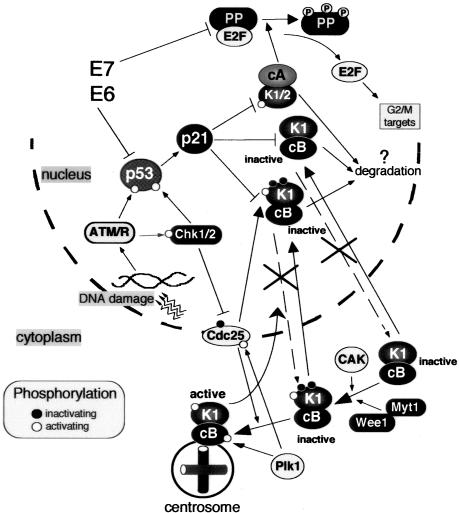
Figure 8.
Role of p21 in establishing irreversible cell cycle arrest in G2 after genotoxic stress. In response to DNA damage, G2 arrest is initiated by Chk1/2-dependent inactivation of Cdc25 family phosphatases (Cdc 25A, B, C), thus preventing activation of cyclin B1-Cdk1. The maintenance of G2 arrest is assured by p21-dependent inactivation of mitotic Cdks (cyclin B1-Cdk1 and cyclin A-Cdk1) and their eventual degradation. Cyclin B1-Cdk1 complexes are maintained in an inactive state by association with p21, which provokes nuclear retention of cyclin B1-Cdk1 and prevents their activation at the centrosome and, possibly, in the cytoplasm and nucleus. By binding to cyclin B1-Cdk1, p21 prevents both activation by CAK-dependent phosphorylation of Thr161 (white circle) and Cdc25-dependent dephosphorylation of Thr14 and Tyr15 (black circles). For the sake of simplicity, Wee1 and CAK, which are predominantly in the nucleus, are placed in cytoplasm. Additionally, p21 inactivates cyclin-Cdk complexes (such as cyclin A-Cdk2) responsible for phosphorylation of pocket proteins (PP; pRB, p107, and p130) during S/G2/M progression. Subsequent accumulation of active pocket proteins blocks expression of genes involved in G2/M progression (Ren et al., 2002; Baus et al., 2003). Inactivation of either p53 or pocket proteins by expression of viral oncogenes (such as HPV16-E6 and HPV16-E7), does not interfere with the cell cycle arrest in G2 but compromises the irreversible cell cycle exit. Black and white circles denote inactivating and activating phosphorylations (P), respectively.