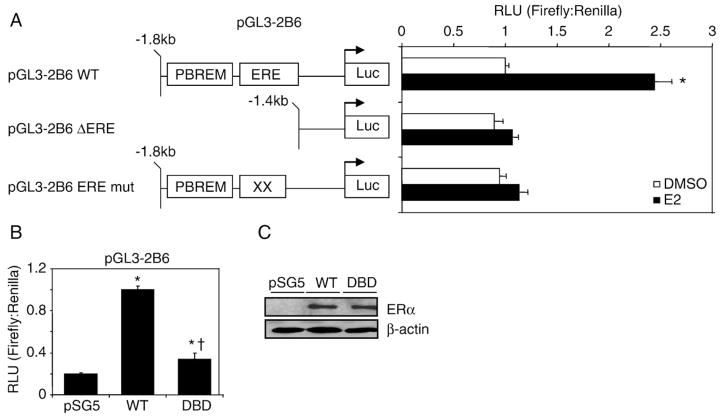
Fig. 4.
ERα-dependent regulation of pGL3-2B6 occurs through the ERE site. (A) HuH-7 cells were transfected with 200 ng of pGL3-2B6, pGL3-2B6_EREmut or pGL3-2B6_ΔERE and 50 ng of pSG5 ERα for 24 h. Cells were treated with either 0.1% DMSO or 10 nM E2 for 24 h prior to luminescence detection. Results shown are means±S.E.M. for five independent experiments. Luciferase activity that is statistically significant (p<0.05 one-way ANOVA) from DMSO pGL3-2B6 is indicated by an asterisk. (B) HuH-7 cells were plated in full serum and transfected with 200 ng of pGL3-2B6 ERE and 100 ng of either pSG5 ERα or pSG5 ERα DBD. Results shown are means±S.E.M. for three independent experiments. Luciferase activity that was significantly different (p<0.05 one-way ANOVA) from pSG5 (vector control) or wild-type ERα are indicated by an asterisk or dagger, respectively. (C) Western blot analysis shows that the wild-type and the mutant receptors were expressed at similar levels.