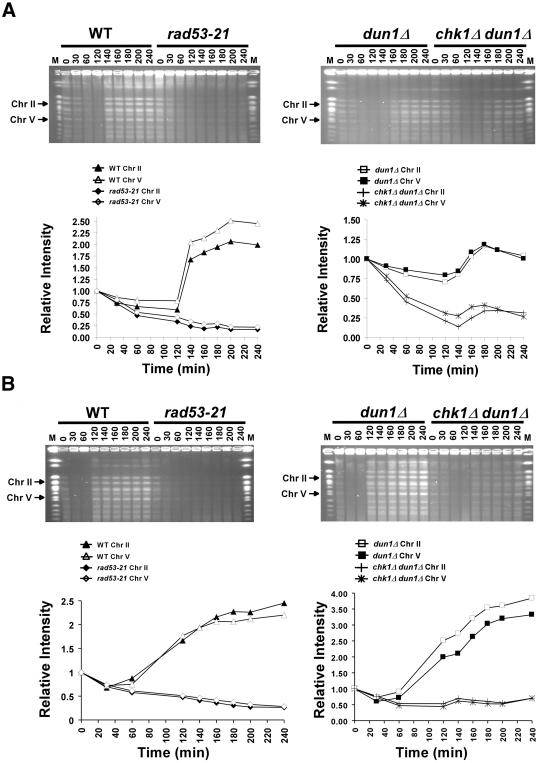
Figure 6.
chk1Δ dun1Δ cells are defective in recovery from replication blocks induced by HU. Wild-type (Y300), dun1Δ (Y578), rad53-21 (Y301), and chk1Δ dun1Δ (Y857) cells were synchronized in G1 with α-factor and then released (at time = 0) into YPD containing 200 mM (A) or 50 mM (B) HU and 10 μg/ml nocodazole. Sixty minutes after release, cells were resuspended in YPD medium with nocodazole. Cells were collected at the indicated times during and after HU treatment, fixed in 70% EtOH, and used to prepare DNA-embedded agarose plugs for PFGE. Chromosomes were visualized by staining the gels with ethidium bromide, and the EtBr signals for chromosomes II and V (arrows) were quantified and graphed. M, molecular weight markers composed of S. cerevisiae chromosomes (New England Biolabs, Beverly, MA). The results shown are representative of five independent repetitions of each experiment. Despite some experiment-to-experiment variability (see Materials and Methods), the greater defect of the chk1Δ dun1Δ cells (relative to dun1Δ cells) was apparent in all trials. Note that the dun1Δ cells in panel A, unlike the wild-type cells, may not have reached a twofold increase in band intensity during the experiment because of this variability, because of residual unresolved DNA structures that prevent some chromosomes from entering the gel after transient treatment with the higher concentration of HU, or both.