Figure 3.
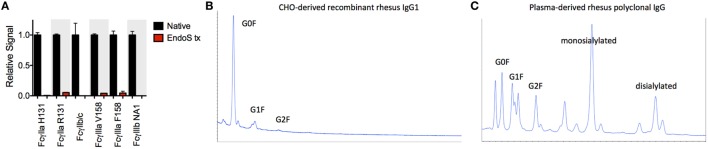
Glycosylation state of Macaca mulatta (MM) immunoglobulin G (IgG) and glycan requirement for binding to low affinity human FcγR. (A) Recombinant monoclonal rhesus macaque IgG (MM IgG1–4) with minor contamination with dimeric IgG and purified polyclonal rhesus macaque plasma IgG (MM pIgG) were either untreated or treated with EndoS (red) leaving a variably fucosylated GlcNac core of the Fc domain glycan. Triplicate measurements at 5 × 10−7 M MM IgG1 in one representative multiplex assay are presented. (B,C) IgG glycosylation as determined by glycan HPLC for MM IgG1 (B) and polyclonal IgG derived from MM serum (C). Peak identities were determined by exoglycosidase digests and glycan controls.