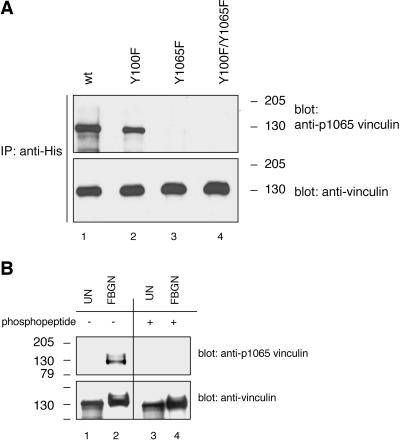
Figure 6.
Phosphorylation site-specific antibody recognizing vinculin when phosphorylated on tyrosine residue 1065 reacts with platelet vinculin. (A) COS-7 cells were cotransfected with a constitutively active c-Src (Y529F) cDNA and with cDNAs encoding for wild-type His-vinculin (wt; lane 1), the His-vinculin point mutants Y100F (lane 2) and Y1065F (lane 3), or the His-vinculin double mutant Y100F/Y1065F (lane 4). The cells were treated and lysed as described in the legend to Figure 3. Lysates containing equal protein amounts were subjected to immunoprecipitation with a mAb to His, and the immunoprecipitates were analyzed by Western blotting and probing with a rabbit polyclonal antiserum raised against a phosphopeptide immunogen mimicking the phosphorylated tyrosine residue 1065 in vinculin (anti-vinculin[pY1065]). The blot was stripped and reprobed with the antibody to vinculin. (B) Unstimulated (UN) and fibrinogen (FBGN)-adherent platelet lysates (15 μg/sample) were probed by Western blotting with the anti-vinculin[pY1065] antiserum that was not treated (lanes 1 and 2) or that was blocked with the phosphopeptide immunogen (lanes 3 and 4). The blot was stripped and reprobed with the antibody to vinculin.