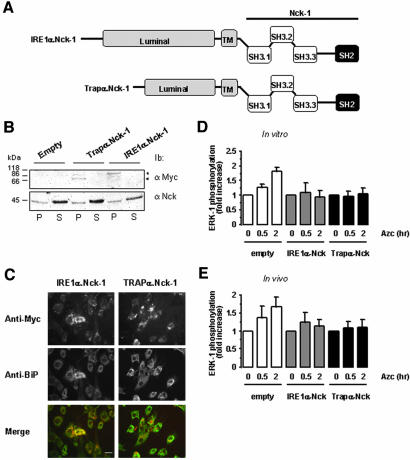
Figure 7.
Forced localization of Nck-1 at the ER membrane affects ER stress-mediated ERK-1 activation. (A) Representative scheme of the fusion proteins IRE1α.Nck-1 and Trapα.Nck-1 used. The luminal and transmembrane domains (gray) of IRE1α or Trapα were fused to the wild-type full-length Nck-1. These constructs were myc-tagged at the C terminus. (B) FR3T3 cells were transfected with pcDNA3, pcDNA3/IREα.Nck-1, or pcDNA3/Trapα.Nck-1, and expression of the respective recombinant protein was tested by immunoblot with anti-myc antibodies in the membrane fraction (P) and in the soluble fraction (S) (top blot). This was compared with the endogenous Nck content in both fractions by immunoblotting with anti-Nck antibodies (bottom blot). A representative immunoblot is shown (n = 3). (C) Immunofluorescence study of FR3T3 cells transfected with pcDNA3/IREα.Nck-1 or pcDNA3/Trapα.Nck-1. Cells were immuno-stained with anti-myc antibodies (top) and anti-BiP antibodies as an ER marker (middle). Costaining was detected by merging of both pictures (bottom). A representative picture is shown (n = 3). Bar, 20 μm. (D) In vitro reconstitution of ERK-1 activation in the presence of RLC was performed as described under Materials and Methods. These experiments were carried out using ER membranes purified from FR3T3 cells transfected with either pcDNA3 (empty) pcDNA3/IREα.Nck-1 or pcDNA3/Trapα.Nck-1 and treated with 10 mM Azc for 0.5 and 2 h. (n = 2, value ± 0.5 variation). (E) ERK-1 phosphorylation in FR3T3 cells transiently transfected either with pcDNA3 (empty) pcDNA3/IREα.Nck-1 or pcDNA3/Trapα.Nck-1 and treated with 10 mM Azc for 0.5 and 2 h (n = 2, value ± 0.5 variation). Lysates were directly immunoblotted with anti-phospho-ERK and anti-ERK-1 antibodies. Immunoblots were quantified by scanning densitometry (n = 2, value ± 0.5 variation).