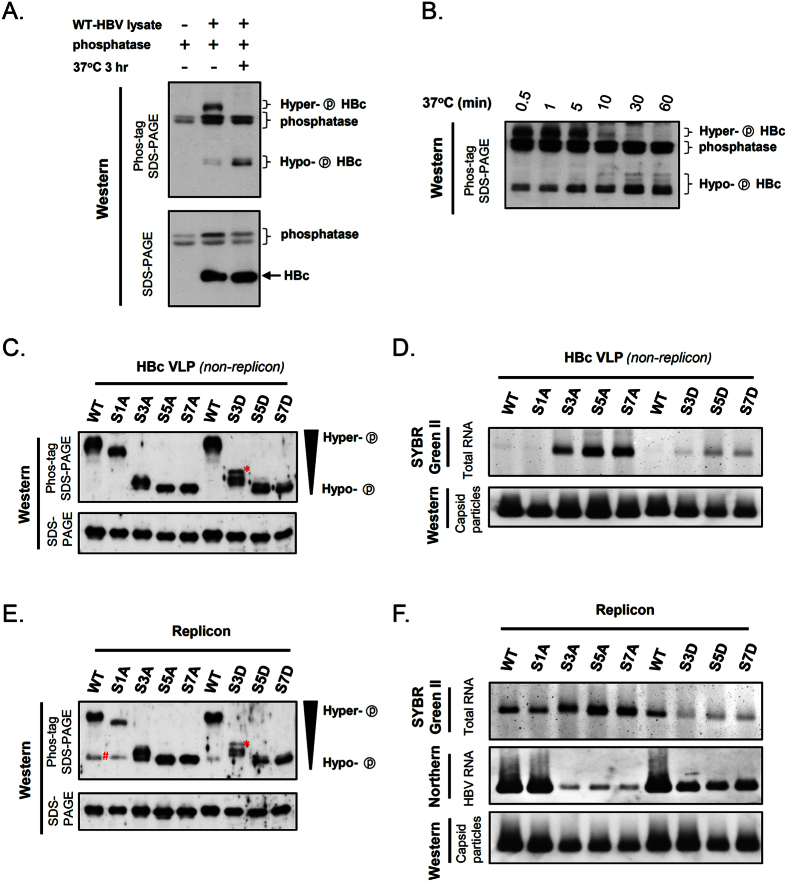
Figure 6. Evaluation of the effect of serine phosphorylation at HBc ARD on capsid assembly and RNA packaging in replicon and non-replicon contexts.
(A) Upper panel: Upon phosphatase treatment, hyper-phosphorylated HBc of a WT-HBV replicon downshifted to a faster-migrating position on the Phos-tag SDS-PAGE. Mobility of phosphoproteins on this gel assay is determined by the degree of phosphorylation62,63,64. Lower panel: In contrast, the phosphorylation status of HBc cannot be resolved by the regular SDS-PAGE. (B) In a time course experiment with phosphatase digestion (0.5 - 60 min), a gradual downshift pattern of WT-HBc protein was monitored on the Phos-tag gel. (C) Upper panel: In the non-replicon context in HuH-7 cells, the phosphorylation status of HBc ARD from WT and serine phosphorylation mutants was assessed by using the Phos-tag gel assay. Lower panel: As an internal control, the same samples were analyzed by regular SDS-PAGE and Western blot. *This band is reproducibly present in S3D, but not in S3A (see Discussion). (D) Upper panel: RNA contents of HBc VLPs (non-replicon) were analyzed by staining with SYBR Green II. Lower panel: Assembled capsids were included as an internal control and visualized by Western blot analysis. (E) In the HBV replicon context in HuH-7 cells, the phosphorylation status of HBc ARD from WT and serine phosphorylation mutants was analyzed by Phos-tag SDS-PAGE. As an internal control, the same samples were analyzed by regular SDS-PAGE and Western blot. *This band is reproducibly present in S3D, but not in S3A (see Discussion). #This band is present only in the replicon system (compare Fig. 6C,E). (F) In the HBV replicon context, HBV capsids were measured by native gel and stained with SYBR Green II (Upper panel), and assayed by Northern blot by using a 3.2 kb HBV-specific probe (Middle panel) and Western blot by using anti-HBc Ab (Lower panel).