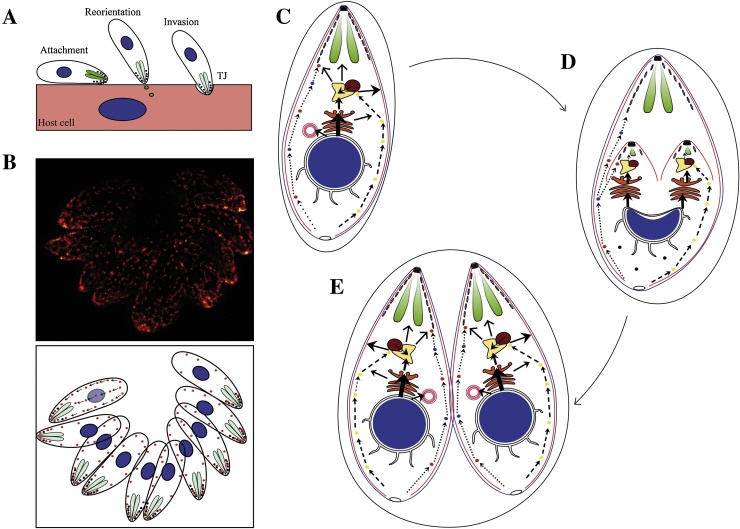
Fig. 2.
Hypothetical model of microneme protein recycling. (A) T. gondii secretes microneme, rhoptry and dense granules proteins during the process of invasion. TJ: tight junction. (B) STED image clearly suggest a highly dynamic movement of microneme organelles in T.gondii. Cartoon representation of the recycling process in the lower panel α-MIC2 antibodies were used for the staining of micronemes in this STED image [35]. (C–D) Parasite replication inside the host cell. (C) Micronemes might be recycled through the posterior end to apical pole (red and blue dots and dotted arrows), however only new synthesised proteins would be transported via endosomes (thick solid arrows). Host proteins and macromolecules ingested by the parasite [18] could be introduced into the parasite using this transport (yellow dots and dashed arrows). Apicoplast proteins are mainly nuclear encoded and post-translationally imported via the secretory system; however, the mechanism by which proteins are transferred from the secretory system to the apicoplast is poorly understood and probably depends on VPS34/VPS15 complex. (D) During endodyogeny microneme, rhoptry and dense granules organelles are synthesized de-novo. The recycled MIC proteins are redirected to the newly formed daughter cells along with host proteins and macromolecules ingested by the parasite (red/blue and yellow dots; dotted and dashed arrows respectively). This model would explain STED images and some conditional mutants, such as VPS11 knockdown and Rab5 overexpression mutants [35], [67] data to date. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)