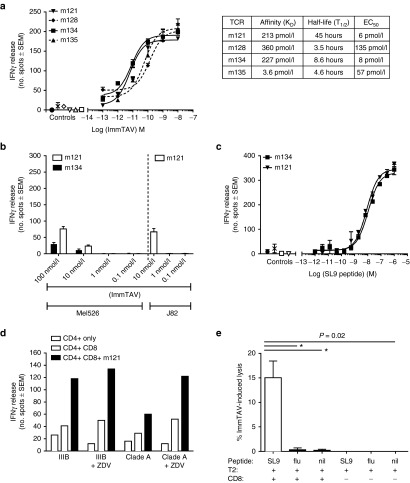
Figure 1.
Biophysical and functional analysis of affinity-matured immune-mobilising monoclonal T-cell receptors against viruses (ImmTAVs) specific for SL9 epitope. (a) Left: Interferon (IFN)-γ responses of CD8+ T-cells from a healthy donor (8 × 104 cells/well) mediated by titrated concentrations of four SL9-directed ImmTAVs (m121, m128, m134, and m135) when cultured with T2 cells pulsed with 10–9 mol/l SL9 peptide (5 × 104 cells/well), quantified by ELISpot. Negative controls for each ImmTAV at 10–9 mol/l were T2 cells pulsed with an irrelevant peptide (open symbols), CD8+ T-cells in the absence of ImmTAV (cross symbol), and T2 cells only (closed circle). Right: Binding parameters (affinity (KD) and half-life (T1/2)) of each of the four ImmTAVs to SL9 peptide, obtained by surface plasmon resonance analysis. (b) Reactivity of ImmTAVs m121 and m134, against Gag SL9-negative, human histocompatibility leukocyte antigen (HLA) -A*0201-expressing human melanoma cells (Mel526) (5 × 104 cells/well). m121 was also tested against malignant human urothelial cells (J82). ImmTAVs were used at concentrations indicated, with CD8+ T-cells from a healthy donor (8 × 104 cells/well). (c). IFN-γ ELISpot assays showing sensitivity of ImmTAVs, m121, and m134 (10–9 mol/l ImmTAV), to T2 cells (5 × 104/well) pulsed with titrated concentrations of SL9 peptide. Healthy donor CD8+ T-cells and controls were as described in a. (d) Response to human immunodeficiency virus (HIV) -infected (IIIB or A isolates) primary CD4+ T-cells (5 × 104 cells/well) by healthy donor CD8+ T-cells (8 × 104 cells/well) when redirected by m121 ImmTAV (10–8 mol/l). Zidovudine (ZDV) (5 μg/ml) was added as indicated, as we observed that this reduced nonspecific IFN-γ release by CD4+ T-cells when infected with HIV in vitro. (e) CFSE-labelled T2 cells were pulsed with 10 μmol/l SL9 peptide, irrelevant (influenza) peptide or unpulsed, then cultured with healthy donor CD8+ T-cells with or without ImmTAV (1 nmol/l) for 24 hours and analyzed by flow cytometry.