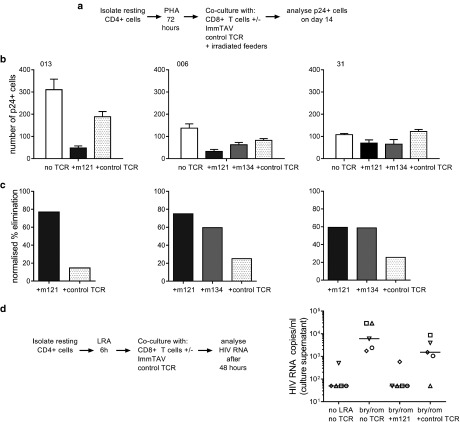
Figure 7.
ImmTAV-mediated clearance of autologous HIV reservoir cells. (a). Schema illustrating assay to quantify ImmTAV-mediated elimination of resting HIV-infected Cd4+ T-cells from ART-treated patients after viral reactivation. CD25-/69-/HLA-DR- CD4+ cells were thoroughly washed after PHA treatment and cultured in duplicate at a density of 3 × 105 cells/well alone, with autologous CD8+ T-cells only or with healthy donor CD8+ T-cells plus ImmTAVs (10–9 mol/l). Irradiated allogeneic PBMC feeders (1.5–3 × 106) were added to all wells. CD8+/CD4+ cell ratios were 1:1 throughout. After 14 days, Gag+ cells were quantified by flow cytometry. (b) Number of Gag+ cells remaining at day 14 of culture (mean of duplicate wells). (c) Percent elimination of Gag+ cells, determined by normalizing to no TCRs. In all subjects, culture supernatants from CD4+ cell-only wells were positive for free p24 Ag and ImmTAV-treated wells were negative (determined by enzyme-linked immunosorbent assay, cut-off 1500 pg/ml). (d) CD25-/69-/HLA-DR- CD4+ cells from five ART-treated HLA-A*0201-positive subjects were thoroughly washed after LRA (bryostatin / romidepsin) treatment and cultured in duplicate with healthy donor CD8+ T-cells (1:1 ratio) ± ImmTAV m121 or control TCR (10–9 mol/l) for a further 42 hours. Supernatants were harvested and viral outgrowth was determined by quantification of HIV RNA, as described in Materials and Methods. LRA, latency-reversing agent; HLA, human histocompatibility leukocyte antigen; ART, antiretroviral therapy; PHA, phytohemagglutinin; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus; PBMC, peripheral blood mononuclear cells; TCR, T-cell receptor.