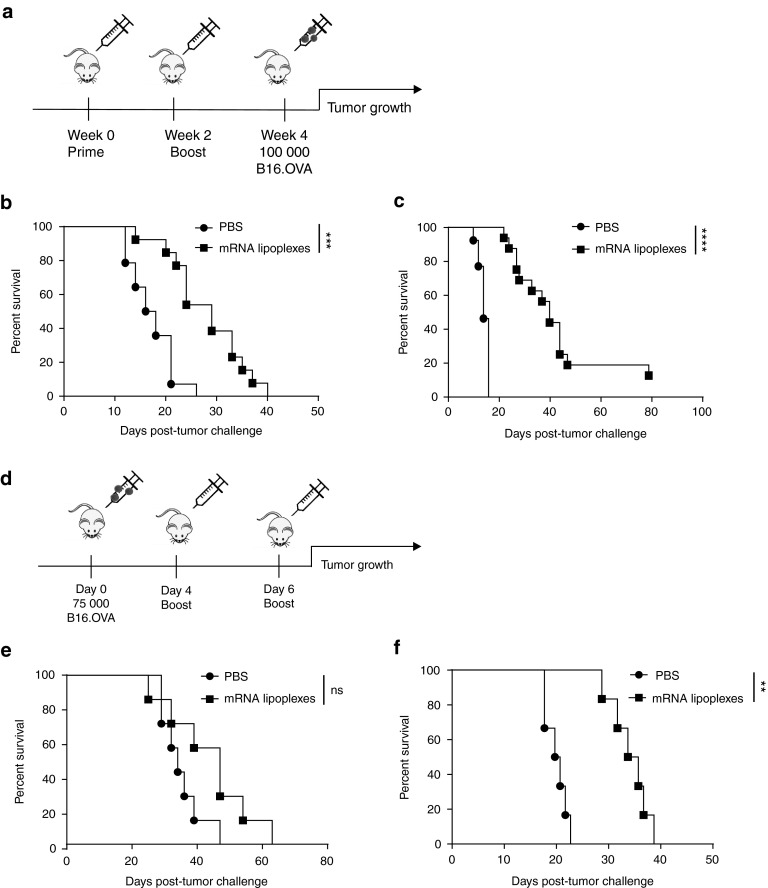
Figure 3.
Impact of type I IFNs on the efficacy of antitumor immunity elicited by mRNA lipoplex vaccination. (a) Prophylactic vaccination scheme. Wild type (WT) mice (b) and Ifnar−/− mice (c) were either mock s.c. immunized (i.e., injected with PBS only) or immunized with 20 μg of mRNA lipoplexes. After 2 weeks, mice were boosted with the same formulation. At week 4, mice were inoculated with 100,000 OVA-expressing B16 melanoma cells. (n = 12–16 mice/group). (d) Therapeutic vaccination scheme. Wild type (WT) mice (e) and Ifnar−/− mice (f) were inoculated with 75,000 B16.OVA melanoma cells. After 4 and 6 days, immunization was performed with similar preparations as in the prophylactic setting. (n = 5–6 mice/group). mRNA lipoplexes = OVA-coding messenger mRNA complexed to DOTAP/DOPE liposomes. **P < 0.01; ***P < 0.001; ****P < 0.0001 (Mantel-Cox log-rank test). PBS, phosphate-buffered saline; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.