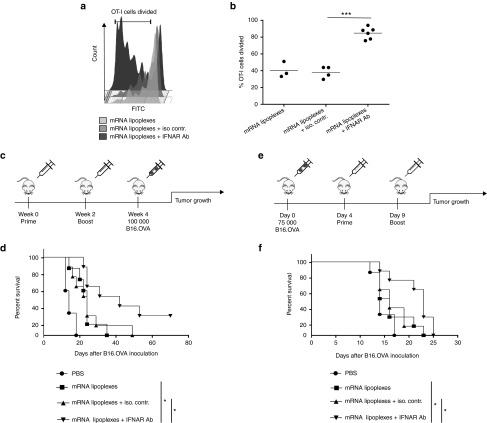
Figure 4.
Antibody-mediated blocking of IFNAR improves the efficacy of the mRNA vaccine evoked antitumor immune response. (a,b) Two days prior to immunization CFSE-labeled OT-I cells were adoptively transferred to wild type (WT) mice. Immunization was performed in the footpad with 10 µg mRNA lipoplexes in the absence or presence of 20 µg IFNAR blocking antibody or isotype control. Four days after immunization inguinal lymph nodes were isolated and CD8+ T cell proliferation was analyzed by flow cytometry. Data are shown as mean of 3–6 mice per group. ***P < 0.001 (Chi-square test). (a) A representative sample out of 3–6 mice each group is presented. (c) Prophylactic vaccination scheme. (d) Wild type (WT) mice were immunized s.c with 20 μg of mRNA lipoplexes in absence or presence of 20 µg of the IFNAR blocking antibody or isotype control. After 2 weeks, mice were boosted with the same formulation. At week 4, mice were inoculated with 100,000 B16.OVA melanoma cells (n = 6–8 mice/group). *P < 0.05 (Mantel-Cox log-rank test). (e) Therapeutic vaccination scheme. (f) Wild type (WT) mice were inoculated with 75,000 B16.OVA melanoma cells. After 4 and 9 days, immunization was performed using 20 μg of mRNA lipoplexes in absence or presence of the IFNAR blocking antibody or isotype control (20 µg) (n = 6–8 mice/group). *P < 0.05 (Mantel-Cox log-rank test). mRNA lipoplexes = OVA- coding messenger mRNA complexed to DOTAP/DOPE liposomes. CFSE, carboxyfluorescein diacetate succinimedyl ester; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.