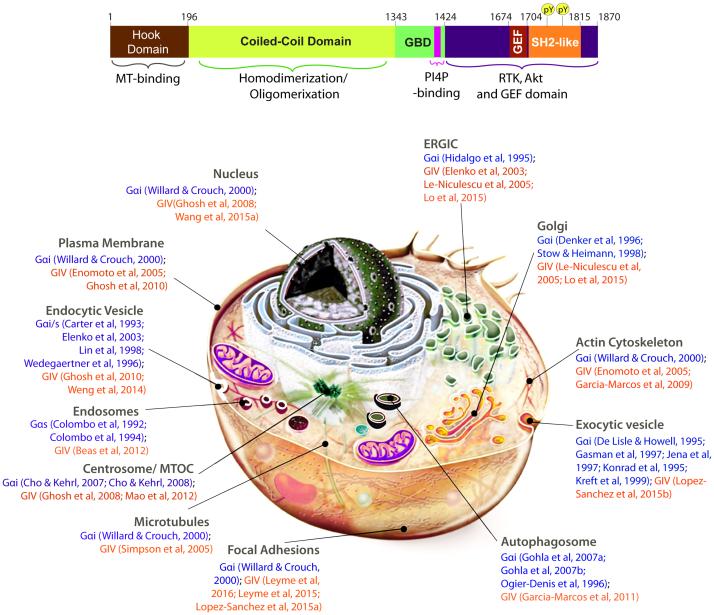
Figure 1. GIV, a multimodular cytosolic protein and GEF for Gαi that localizes to and functions at various subcellular locations.
Top: Bar diagram showing the various known functional modules of full-length GIV. Residue numbers marking the boundaries of each domain are shown. The C-terminal domain features multiple short patches of functional motifs and modules that enable GIV to bind other proteins and transduce signals. Among them, a C-terminally located GEF motif (Garcia-Marcos et al, 2010; Garcia-Marcos et al, 2009) binds and activates Gαi proteins by triggering nucleotide exchange. Just downstream of the GEF motif is a SH2-like domain (Lin et al, 2014) which recognizes and folds upon binding phosphotyrosine ligands on the cytoplasmic tails of multiple RTKs. GIV is also a substrate of multiple TKs (Lin et al, 2011). Phosphorylation of GIV at two tyrosines (pY1764 and pY1798) by multiple receptor and non-receptor protein tyrosine kinases generates two docking sites for SH2-adaptor containing proteins. Bottom: Schematic of a cell with various subcellular organelles and locations where both G proteins and GIV are known to locate. Numbers denote citations that provide evidence for such localizations. Published work demonstrates that GIV and its GEF function is functional at most, if not all of these locations.