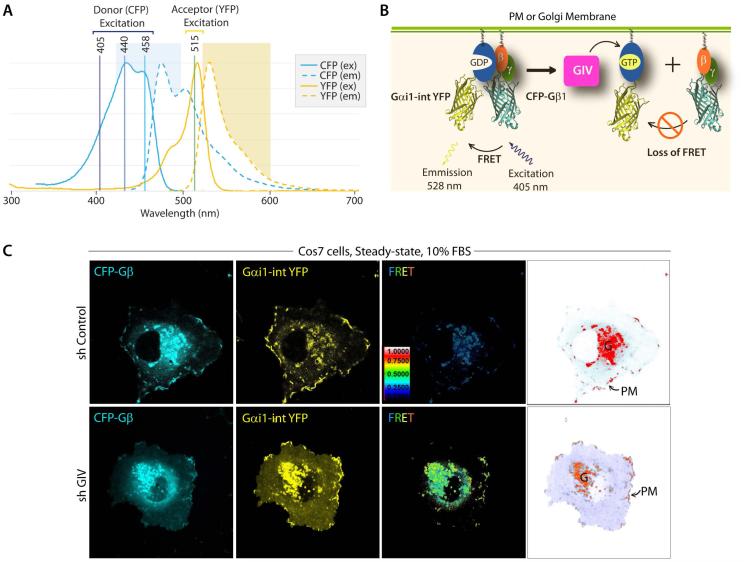
Figure 5. Measurement of GIV-dependent Gi activation by FRET imaging.
A. Illustration of spectral overlap integral between the fluorescence spectrum of cyan-fluorescent protein (CFP), and the absorption spectrum of yellow-fluorescent protein (YFP). CFP and YFP are mutants of GFP. The 3 lasers that are commonly used for excitation of donor (CFP) and direct activation of acceptor (YFP) are indicated. The 405 nm laser selectively excites CFP with little or no excitation of YFP. B. Schematic for the internally tagged Gαi1-(int)YFP, CFP-Gβ1 and untagged Gγ2 constructs used as paired FRET probes. When associated as a Gαi1-βγ heterotrimer, FRET can be observed. Activation of Gi by GIV-GEF (magenta box) triggers the dissociation of Gαi1-(int)YFP from CFP-Gβ1γ2 heterodimers, resulting in loss of FRET. Thus, high FRET indicates that a majority of Gi1 is inactive, whereas a loss of FRET (ligand-dependent) or low FRET (at steady-state) indicates that a significant pool of Gi1 is active. C. Control (sh Control) and GIV-depleted (sh GIV) COS7 cells were cotransfected with Gαi1-(int)YFP, CFP-Gβ1 and Gγ2 (untagged) and live cells were analyzed by FRET imaging at steady-state, in the presence of 10% serum. From left to right, representative freeze-frame CFP, YFP and FRET images are shown. FRET image panels display intensities of acceptor emission due to efficient energy transfer in each pixel. Right most panel shows the different membranes (in red) that are imaged simultaneously to analyze activation of Gi at both sites-- 1) internal membranes in the perinuclear region, which was largely determined to be the Golgi (G), and 2) peripheral PM ruffles. In control cells, FRET is low (i.e., Gi1 is active) at both locations, whereas in GIV-depleted cells FRET is high at both locations. These observations have been described in details by Lo I et al (Lo et al, 2015).