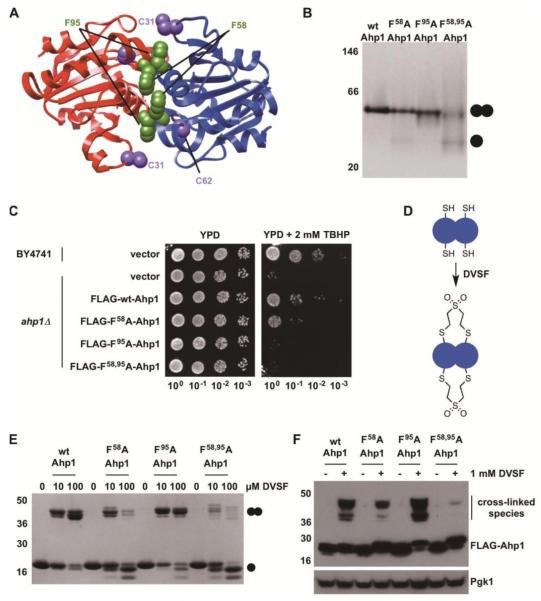
Figure 5. Disruption of the Redox Center at the Dimer Interface of Ahp1 Compromises Cellular Defense against Organic Peroxides and Impairs Cross-Linking by DVSF.
(A) Structure of Ahp1 depicting catalytic Cys residues (C62 (peroxidatic Cys) and C31 (resolving Cys)) and conserved Phe residues at the dimer interface. Protein structures were generated with Chimera (https://www.cgl.ucsf.edu/chimera/) using PDB 4DSR. (B) Purified recombinant Ahp1 (wild-type (wt) or dimer interface variants, 20 μM) were resolved on native-PAGE and detected with Coomassie blue to determine oligomeric state. (C) Wild-type (wt) or mutant forms of Ahp1 were expressed in ahp1Δ yeast. Serial dilutions of these cultures and corresponding controls were grown on YPD medium containing 2 mM TBHP for 48 h at 30°C to determine the effect of dimer interface disruption on oxidant defense. (D) Scheme depicting proposed inter-subunit cross-linking in Ahp1 by DVSF. (E) Purified Ahp1 proteins (10 μM) were treated with increasing concentrations of DVSF for 3 h at 37°C, prior to electrophoresis on SDS-PAGE and detection by staining with Coomassie blue. (F) Log-phase yeast cells expressing Ahp1 variants were treated with 1 mM DVSF for 1 h at 30°C. Protein lysates (10-20 μg) were resolved by SDS-PAGE, transferred to PVDF membrane, and detected with an antibody against the FLAG-tag or Pgk1 (loading control) via Western blot. Results for all experiments are representative of three independent trials.