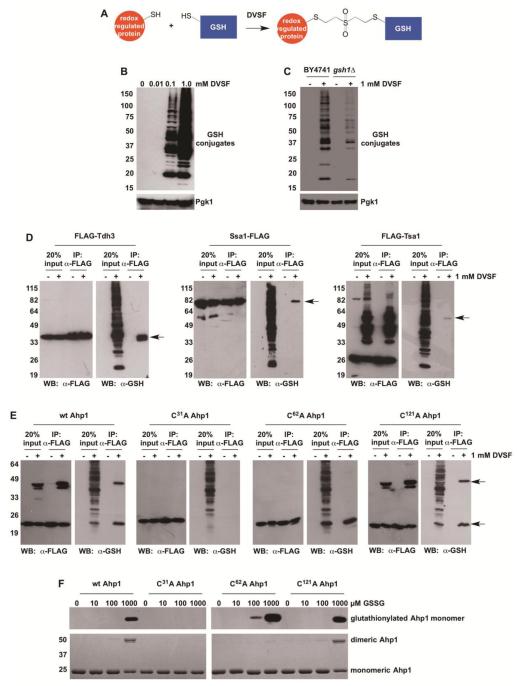
Figure 6. Cross-Linking of GSH to Redox-Active Proteins in Cells Treated with DVSF.
(A) Scheme depicting GSH cross-linking to putative DVSF targets. (B) Log-phase yeast cells (BY4741) were treated with increasing doses of DVSF for 1 h at 30°C. Protein lysates (10 μg) were resolved by SDS-PAGE, transferred to PVDF membrane, and detected with an antibody against the FLAG-tag or Pgk1 (loading control) via Western blot. (C) Log-phase yeast cells (BY4741 or gsh1Δ) were treated with 1 mM DVSF for 1 h at 30°C. Protein lysates (10 μg) were resolved by SDS-PAGE, transferred to PVDF membrane, and detected with an antibody against the FLAG-tag or Pgk1 (loading control) via Western blot. (D and E) Cells expressing FLAG-tagged proteins were treated with 1 mM DVSF for 1 h at 30°C. FLAG-tagged proteins were immunoprecipitated from cell lysates (100 μg). Western blots were conducted to determine isolation of FLAG-tagged protein and its conjugation to GSH. Arrows point to protein-glutathione conjugates. (F) Purified Ahp1 variants (10 μM) were incubated with varying concentrations of GSSG for 20 min, prior to alkylation of free cysteines with N-ethylmaleimide and resolution by non-reducing SDS-PAGE. S-glutathionylation was monitored by Western blot. Ahp1 levels and oxidation were visualized with Coomassie blue. Results are representative of two-three independent experiments.