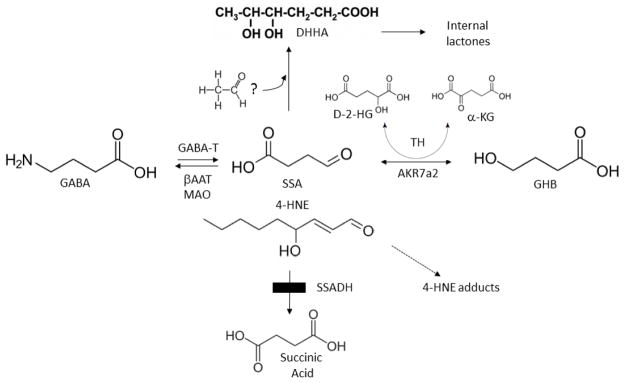
Fig. 1. Schematic diagram of GABA metabolism and metabolic abnormalities in aldh5a1−/− mice.
4-Aminobutyric acid (also γ-aminobutyric acid; GABA) is normally metabolized to succinic semialdehyde via GABA-transaminase (GABA-T), and subsequently forms succinic acid via succinic semialdehyde dehydrogenase (SSADH), the defect in SSADH deficiency (SSADHD; box). Multiple metabolites are elevated in tissues of aldh5a1−/− mice, and in physiological fluids derived from patients with SSADHD. These include: γ-hydroxybutyrate (GHB; produced from succinic semialdehyde (SSA) in a reaction catalyzed by aldo-keto reductase 7a2, AKR7a2); D-2-hydroxyglutaric acid (D-2-HG; produced in a reaction catalyzed by nicotinamide-independent D-2-hydroxyglutaric transhydrogenase (TH), which stoichiometrically interconverts SSA and GHB coupled to the interconversion of D-2-HG with α-ketoglutarate (a-KG)), succinic semialdehyde (SSA); and 4,5-dihydroxyhexanoic acid (DHHA; no enzyme reaction has been identified that catalyzes the formation of DHHA, but it may be derived from SSA condensation with an “activated” two carbon species, perhaps within the pathway of oxidative phosphorylation [53]). In addition to GABA-T, monoamine oxidase (MAO) and β-alanine aminotransferase (βAAT) can also metabolize GABA. Also shown is 4-hydroxy-2-nonenal (4-HNE), which is metabolized to 4-HNE acid in brain in a reaction catalyzed by SSADH. Note the structural similarities of 4-HNE and SSA.