Abstract
Background
Clinical characteristics of isolated, idiopathic cervical dystonia such as onset site and spread to and from additional body regions have been addressed in single-site studies with limited data and incomplete or variable dissociation of focal and segmental subtypes.
Objectives
To characterize clinical characteristics and demographics of isolated, idiopathic cervical dystonia in the largest standardized, multicenter cohort.
Methods
The Dystonia Coalition, through a consortium of 37 recruiting sites in North America, Europe and Australia recruited 1477 participants with focal (60.7%) or segmental (39.3%) cervical dystonia on examination. Clinical and demographic characteristics were evaluated in terms of the body region of dystonia onset and spread.
Results
Site of dystonia onset was: a) focal neck only (78.5%), b) focal onset elsewhere with later segmental spread to neck (13.3%), and c) segmental onset with initial neck involvement (8.2%).Frequency of spread from focal cervical to segmental dystonia (22.8%) was consistent with prior reports, but frequency of segmental onset with initial neck involvement was substantially higher than 3% previously reported. Cervical dystonia with focal neck onset, more than other subtypes, is associated with spread and tremor of any type. Sensory tricks were less frequent in cervical dystonia with segmental components, and segmental cervical onset occurred at an older age.
Conclusions
Subgroups had modest but significant differences in the clinical characteristics that may represent different clinical entities or pathophysiologic subtypes. These findings are critical for design and implementation of studies to describe, treat, or modify disease progression in idiopathic isolated cervical dystonia.
Keywords: Spasmodic torticollis, focal, segmental, geste antagoniste, neck
Introduction
Isolated, idiopathic dystonia (previously known as primary dystonia) includes a heterogeneous group of disorders characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both.1 What factors differentiate subtypes of dystonia and which different pathological mechanisms influence clinical features or variability in therapeutic response remain to be determined.2 In an attempt to study demographic and clinical characteristics, previous efforts focused on increasingly homogenous subgroups to clarify demographics and clinical characteristics of isolated, idiopathic cervical dystonia (CD).3-10 However, these smaller single-site studies did not distinguish CD that began in the neck from that which started elsewhere and spread to the neck.
Most reports of CD have focused on dystonia that begins in the neck and may spread to contiguous body parts.11-16 Occasionally, CD begins as a component of segmental dystonia17, 18 or develops after dystonia emerged in another body part.10,14 Several studies report that site of onset, presence of tremor, disease duration, family history and age of onset affect the risk of spread of dystonia to other body parts.11, 14-16, 19-21 These clinical and demographic features are critical for understanding disease progression and, potentially, response to treatment. Previous large studies of CD either focused mostly on genetic aspects,22-26 or did not address progression.27 Thus, none of these studies distinguished various clinical characteristics of CD across onset type including focal (with or without later spread), segmental, or appearance after dystonia began elsewhere. These clinical characteristics may be essential for design and implementation of future studies that aim to describe, treat or modify disease progression in those with idiopathic isolated CD 28.
The primary purpose of this work was to characterize the clinical characteristics and demographics of isolated, idiopathic CD in the largest standardized, multicenter cohort reported to date. We focused on CD since this is the most common isolated focal dystonia2, 29 and represents the largest group in the Dystonia Coalition clinical database (70%). This large sample permits examination of demographic and clinical characteristics across the entire phenotypic spectrum of CD, including focal and segmental forms and spread to or from other body regions.
Methods
Data were obtained from the Dystonia Coalition clinical database for Project 1 (Natural History and Biorepository subprojects). Thirty-seven institutions across 7 countries contributed to the database using a common consent, data intake form and examination protocol. All subjects gave written informed consent at the recruiting site according to the Declaration of Helsinki and The Common Rule. Analysis of de-identified aggregate data was further exempted by the Emory University Human Subjects Review Board and the Human Research Protection Office at Washington University. All data from January 5, 2011 (study onset) through September 30, 2015 were used. We only included data from the first visit if a participant had additional follow up visits.
Eligibility for Dystonia Coalition studies required a diagnosis of isolated, idiopathic dystonia and age greater than 17 years. A standard clinician administered questionnaire (supplemental figure 1) and video protocol was collected for each participant at the time of enrollment; the latter is kept in a video repository available to investigators with institutional review board and Dystonia Coalition approval.30 Specifically, the questionnaire accounted for each participants’ account regarding dystonia onset site and improvement of dystonia (not tremor) following alcohol ingestion. Thus, these particular data were collected retrospectively. All participants were evaluated by movement disorders specialists either in person or by video to identify all body regions affected by dystonia, to complete rating scales, and to identify presence or absence of tremor. Specifically, the examiner noted involvement of each affected body region (upper face, lower face, jaw, tongue, neck, shoulder, upper arm, hand, trunk, pelvis, upper leg, foot), documented the affected side of the body, and indicated presence or absence of tremor in each of the body regions. In addition, video recordings included each participant's demonstration of an attempted sensory trick, and this remains documented in the Dystonia Coalition video repository.30 Symptom severity was rated using the Global Dystonia Rating Scale (GDRS) and Burke-Fahn Marsden rating (BFM) for all enrolled participants. Final classification of dystonia into focal, segmental, multifocal or generalized was based on documented examination findings.1, 12 Shoulder involvement was included as a component of focal CD. Tremor affecting dystonic regions was distinguished from tremor affecting regions without dystonia.31, 32 All participants had cervical dystonia; some had tremor of the neck and some had tremor of other body parts that may or may not have had dystonia. Tremor alone was not rated as dystonia in the absence of dystonic posture of a body region. All examinations were performed at least two months after botulinum toxin injections or when symptoms returned. In this study, exclusion criteria include patients with CD secondary to known causes such as medication-induced dystonia, parkinsonian syndromes, and those with orthopedic procedures that may affect neck movement. For data presented here, videos were reviewed and new exam ratings generated only if clarification regarding location or type of dystonia was necessary, otherwise exam ratings by recruiting investigators were used. Details regarding the Dystonia Coalition can be found at https://www.rarediseasesnetwork.org/cms/dystonia/.
All statistics were completed with IBM SPSS Statistics for Windows, Version 23.0. Two-tailed unpaired t-tests and one-way analysis of variance (ANOVA) were used to test group differences in continuous data, including age of onset, dystonia duration, GDRS, and BFM score comparison. When significance was demonstrated by ANOVA, post-hoc Tukey-Kramer statistics were used for pairwise comparisons. Multinomial logistic regression was performed to account for covariates when assessing the main effect association of clinical characteristics with dystonia subtype.33 The first regression model contained dependent variables (dystonia subtypes), independent variables (clinical characteristics: presence of sensory trick, presence of tremor affecting a dystonic region, presence of tremor affecting a non-dystonic region) and covariates (sex, age at onset, duration of dystonia, and neck dystonia severity as measured by GDRS and BFM). Regression was repeated with each dependent variable as reference. Response of dystonia to alcohol was omitted from the first regression model due 724 missing cases (only 51% of all participants responded with a known effect to alcohol). We then repeated a similar multinomial logistic regression model, including response of dystonia to alcohol included as an independent variable, which resulted in 753 valid cases. Chi-square tests were used to test differences in frequency distribution of dystonia response to alcohol across tremor groups (tremor affecting a dystonic body region or tremor affecting a non-dystonic body region) with the null hypothesis of no association following Bonferoni correction. All statistics were considered significant with p<0.05.
Results
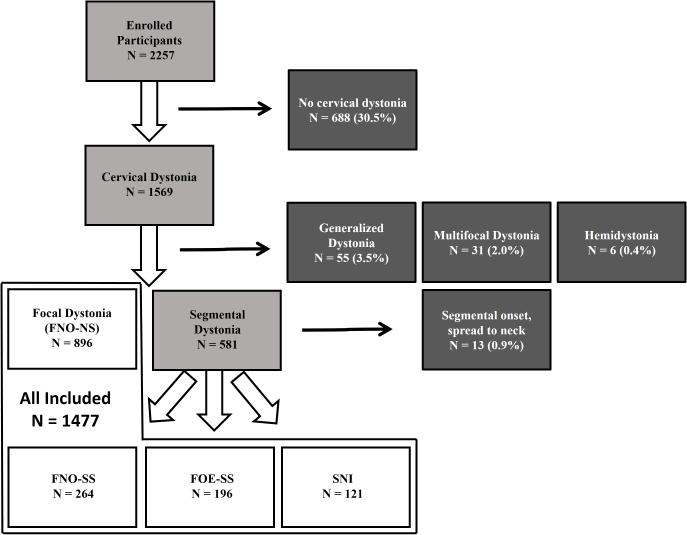
Data from 1582 participants with CD were extracted from a pool of 2257 consecutively enrolled participants in the Dystonia Coalition project 1. Subgroup analyses were performed on 1477 participants with focal (60.7%) and segmental (39.3%) dystonia at study enrollment (Figure 1). This analysis excluded subgroups with numbers too small for meaningful interpretation (see below). Thirty-seven international institutions in Australia (1), Europe (4), and North America (3 in Canada, 29 in the USA) recruited and enrolled participants for this study. Median contribution per institution of those with cervical dystonia was 25.5 participants (interquartile rage, 42; range 4-196). Regarding intercontinental recruitment, 1337 participants were recruited in North America, 226 in Europe, and 17 in Australia.
FIG. 1.
Study workflow (light gray boxes) to included (white boxes) and excluded participants (dark boxes). FNO-NS: focal neck onset, no spread; FNO-SS: focal neck onset, segmental spread; SNI: segmental onset with neck involvement, FOE-SS: focal onset elsewhere, segmental spread.
Demographic data for all subjects with CD are reported in Table 1 (column 1). The mean age for all participants at study enrollment was 59.8 ± 12.4 years; 86.8% were right-handed and 2.5% were ambidextrous.
Table 1.
Demographic and clinical characteristics by dystonia site of onset and spread
| All |
Focal |
Segmental |
|||
|---|---|---|---|---|---|
| FNO-NS |
FNO-SS |
SNI |
FOE-SS |
||
| Number of subjects | 1477 | 896 | 264 | 121 | 196 |
| Right handed | 1283 (86.9%) | 789 (88.1%) | 228 (86.4%) | 105 (86.6%) | 164 (83.7%) |
| Woman | 1102 (74.6%) | 661 (73.8%) | 205 (77.7%) | 91 (75.4%) | 144 (73.6%) |
| Sensory trick | 1015 (68.7%) | 653 (72.9%)* | 189 (71.6%)* | 78 (64.2%) | 101 (51.4%)* |
| Response to alcohol | 263 (17.8%) | 158 (17.6%) | 66 (25.0%)* | 13 (10.4%)* | 28 (14.4%) |
| Tremor of dystonic region | 798 (54%) | 467 (52.1%) | 175 (66.1%)* | 67 (55.2%) | 88 (45%)* |
| Tremor of nondystonic region | 134 (9.1%) | 88 (9.8%)* | 22 (8.5%)* | 9 (7.8%)* | 15 (7.5%) |
| Age of Onset (years) | 45.8 ± 14.2 | 45.2 ± 13.5 | 45.0 ± 14.3 | 49.7 ± 15.0* | 46.6 ± 15.9 |
| Dystonia Duration (years) | 14.6 ± 12.0 | 14.3 ± 11.8 | 17.6 ± 13.0* | 11.0 ± 11.0* | 14.3 ± 11.8 |
| GDRS total | 8.5 ± 6.0 | 6.2 ± 3.7* | 10.8 ± 5.9* | 12.9 ± 8.7* | 12.3 ± 7.5* |
| GDRS neck | 4.8 ± 2.2 | 5.0 ± 2.1 | 5.1 ± 1.9 | 4.5 ± 2.2* | 3.5 ± 2.2* |
| BFM total | 7.3 ± 5.3 | 4.6 ± 3.2* | 7.6 ± 6.1* | 9.0 ± 7.7* | 10.3 ± 8.0* |
| BFM neck | 4.5 ± 2.1 | 4.6 ± 2.1 | 4.8 ± 1.9 | 2.9 ± 2.2* | 3.8 ± 2.1* |
Significant associations across all onset subtypes (FNO-NS, focal neck onset-no spread; FNO-SS, focal neck onset-segemental spread; SNI, segmental onset with neck involvement, FOE-SS, focal onset elsewhere-segmental spread). GDRS = Global dystonia rating scale; BFM = Burke-Fahn-Marsden scale
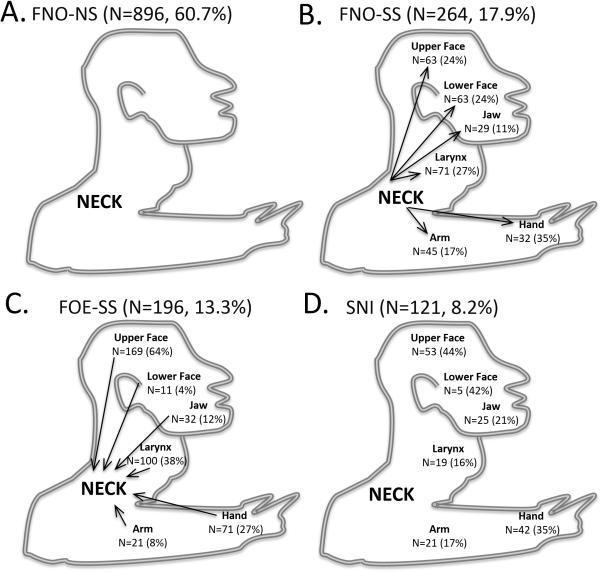
Focal neck onset was reported by 1160 participants (78.5%), and 896 of these (77.2%) remained focal with no spread while 264 (22.8%) later spread to a contiguous body region, thereby defining segmental dystonia. Segmental cervical onset was reported in 121 (8.2%) and focal onset elsewhere (in another body region) that later spread to neck in 196 (13.3%). Thirteen subjects had segmental onset without neck involvement (that subsequently spread to neck), but were excluded from the final pool of 1477 participants since this number was too small for meaningful interpretation. The excluded subgroup included 8 with cranial (5 with blepharospasm-oromandibular, 2 with blepharospasm-lingual-oromandibular, and 1 with blepharospasm-lingual dystonia), 3 with cranial-laryngeal, and 2 with cranial-upper limb segmental onset that later spread to involve the neck. Thus, 86.7% of all participants with focal or segmental CD reported symptom onset involving the neck. Data regarding onset site, body regions affected at onset (segmental cervical onset and focal onset elsewhere that later spread to neck), and examination findings at intake (focal neck onset with segmental spread) are displayed in Figure 2.
FIG. 2.
Cartoon depiction of body regions involved at onset and dystonia spread in all participants for A. FNO-NS5focal neck onset, no spread, B. FNO-SS5focal neck onset, segmental spread, C. FOE-SS5Focal onset elsewhere with segmental spread to neck, and D. SNI5segmental neck involvement without spread.
Age of onset, dystonia duration, global dystonia rating scale total, global dystonia rating scale neck, Burke-Fahn-Marsden total, and Burke-Fahn-Marsden neck scores significantly differed across onset types. Post-hoc comparisons revealed significantly older age of onset and shorter dystonia duration in segmental cervical onset compared to all other subgroups; the focal neck onset with segmental spread group had a longer dystonia duration. Global dystonia rating scale and Burke-Fahn-Marsden total scores also significantly differed between focal neck onset and either segmental cervical onset or focal onset elsewhere that later spread to neck. Global Dystonia rating scale and Burke-Fahn-Marsden neck scores differed across all three groups. For those with segmental dystonia (i.e. focal neck onset with segmental spread, segmental cervical onset, focal onset elsewhere that later spread to neck) and tremor affecting a dystonic body region (N=798), dystonic body regions include the upper face (14.7%), lower face (12.9%), upper arm (8.6%), hand (20.1%), tongue (1.6%), jaw (5.5%), and larynx (13%). In the same group, tremor affected the upper face (2.2%), lower face (2.5%), upper arm (5.4%), hand (14.6%), tongue (0.3%), jaw (1.8%), and neck (92.6%). In contrast, for those with segmental dystonia and tremor affecting non-dystonic regions (N=134), dystonia affected the upper face (12.6%), lower face (13.4%), upper arm (2.4%), hand (3.1%), tongue (3.1%), jaw (6.3%), and larynx (14.2%). In this group, tremor affected the upper face (7.1%), lower face (11.0%), upper arm (22.0%), hand (81.9%), tongue (3.1%), and jaw (8.7%). Any hand tremor (dystonic or other) was present in 15.3% of all participants (regardless of the body region affected by dystonia), and significantly associated with onset site and spread (χ2 = 49.1, p<0.01). Specifically, hand tremor was less frequent in focal neck onset with no spread and more in focal neck onset with segmental spread and segmental cervical onset subgroups. Duration of dystonia was significantly shorter for those enrolled with segmental cervical onset and longer in those with focal neck onset with segmental spread compared to all other subgroups. Table 1 summarizes demographic and clinical characteristics for the onset type subgroups. Cross-sectional multinomial logistic regression (excluding the independent variable response of dystonia to alcohol) of the association between clinical characteristics (excluding response of dystonia to alcohol) and dystonia subtype indicated statistically significant covariate contributions to the model. Specifically, dystonia duration (X2 = 13.8, p < 0.01), global dystonia rating scale total (X2 = 177.6, p < 0.01), global dystonia rating scale neck (X2 = 69.8, p < 0.01), Burke-Fahn-Marsden total (X2 = 53.2, p < 0.01), and Burke-Fahn-Marsden neck (X2 = 57.6, p < 0.01) contributed but not age at dystonia onset, handedness and sex. The Nagelkerke R2 indicated that the model accounted for 61.4% total variance. Presence of sensory trick, tremor of a non-dystonic body region, and tremor of a dystonic region were associated with dystonia subtype; significant associations are summarized in table 2. In summary, sensory trick was more likely in focal neck onset with no spread (Wald = 12.4, p <0.01) and focal neck onset with segmental spread (Wald = 8.5, p <0.01) compared to focal onset elsewhere that later spread to neck, tremor of a dystonic region was more likely in focal neck onset with segmental spread than focal onset elsewhere that later spread to neck (Wald = 9.6, p < 0.01), and tremor of a nondystonic region was more likely in focal neck onset with no spread than focal neck onset with segmental spread (Wald = 23.9, p < 0.01), segmental cervical onset (Wald = 29.7, p < 0.01), and focal onset elsewhere that later spread to neck (Wald = 7.5, p < 0.01). A separate multinomial logistic regression including response of dystonia to alcohol resulted in significant association with focal neck onset with segmental spread and segmental cervical onset , where response of alcohol to dystonia was more likely in focal neck onset with segmental spread (OR=1.3, Wald = 6.4, p < 0.01). There was no association of dystonia response to alcohol and type of tremor (i.e. tremor of a dystonic body region or tremor of a non-dystonic body region, X2=2.0, p = 0.16).
Table 2.
Significant associations of clinical characteristics with dystonia site of onset
| Sensory trick |
Tremor of dystonic region |
Tremor of nondystonic region |
|---|---|---|
| FNO-NS : FOE-SS | FNO-SS : FOE-SS | FNO-NS : FNO-SS |
| OR=2.4 (CI: 1.5-3.9) | OR=2.2 (CI: 1.3-3.5) | OR=1.4 (CI: 0.9-2.0) |
| FNO-SS : FOE-SS | FNO-NS : SNI | |
| OR=2.0 (CI: 1.3-3.2) | OR=5.7 (CI: 3.0-10.5) | |
| FNO-NS : FOE-SS | ||
| OR = 2.5 (CI: 1.3-4.7) |
*Significant associations (exponential logistic coefficient [odds ratio, OR] with confidence intervals (CI)) for clinical characteristics and dystonia subtype. FNO-NS, focal neck onset-no spread; FNO-SS, focal neck onset-segemental spread; SNI, segmental onset with neck
Discussion
Using the latest consensus criteria1, we report demographic and clinical characteristics from the largest multicenter, international cohort of patients to date with CD. Of those enrolled with focal or segmental dystonia that included CD, the majority reported only neck involvement at onset (focal neck onset), of which 77.2% did not have segmental spread 14.3±11.8 years after onset; whereas 22.8% eventually spread (focal neck onset with segmental spread). The frequency of focal neck onset with segmental spread supports prior reports,11, 13, 14, 16, 34 but accounts for just 45% of participants with segmental CD. The remaining participants with segmental CD either started with segmental cervical onset, or started focally in another body region (focal onset elsewhere that later spread to neck). The former group (8.2% of all participants) is higher than the 3% previously reported.16 The current data demonstrate that clinical characteristics of CD, including presence of an effective sensory trick, response of dystonia to alcohol and presence of tremor affecting dystonic regions differ based on onset site and spread, and thus may represent distinct clinical entities. Specifically, CD with focal neck onset and segmental spread may be more likely to respond to sensory tricks, and involve tremor any type than CD with focal onset elsewhere and segmental spread to the neck. Sensory tricks are less frequent in CD with segmental components, and segmental cervical onset tends to occur at an older age. These findings address the issue of spread not addressed in a subset of similar participants previously reported in a study focusing on genetics27 and are particularly critical for design and implementation of any studies to describe, treat or modify disease progression in those with idiopathic isolated CD.
Cervical Dystonia: Demographics
Demographic characteristics of CD depended upon onset region and spread. The current data support prior studies reporting predominance in women and the typical age of onset, about 42 years-old.7, 8, 10 Age of onset in the segmental cervical onset group appears higher and may reflect the large representation (55%) in this group of participants with blepharospasm. Blepharospasm is a dystonia subtype with older age of onset than CD14 but little is known regarding the frequency in which blepharospasm begins focally compared to beginning as segmental dystonia.
Clinical characteristics based on onset site and spread
Focal and segmental subtypes account for 94.1% (N=1477) of all study participants with CD in our cohort. As a group, those with focal or segmental CD report an effective sensory trick (68.7%), response of dystonia to alcohol ingestion (17.8%), and tremor affecting a dystonic region (54.0%) or non-dystonic region (9.1%). Clinical traits (specifically presence of sensory trick, response of dystonia to alcohol and tremor affecting dystonic regions) differed within focal and segmental subtypes based on onset and spread characteristics.
Sensory trick
With regard to differences in specific clinical characteristics, the percentage of subjects reporting effective sensory tricks differed based on dystonia subtype, but this appeared driven by lower frequencies in focal onset elsewhere that later spread to neck subgroups compared to focal neck onset with segmental spread and focal neck onset with no spread. This indicates that effectiveness of a sensory trick in CD may reflect, in part, onset site in the neck rather than dystonia distribution or spread. Several factors likely contribute to the discrepancy between the current data and prior studies examining sensory tricks in CD.6 First, the current data reflect participant surveys rather than examination findings. Project 2 of the Dystonia Coalition focused on 154 people with CD and identified sensory tricks in 83% of participants based upon investigator review of videos35 suggesting that patients themselves may under-report this phenomenon. Second, successful sensory tricks are reported less in those with more body regions affected, with the exception of focal neck onset with segmental spread. Thus, while the former study focuses on participants with focal neck onset with no spread and focal neck onset with segmental spread, one might anticipate higher reported rates of a sensory trick in a smaller sample size.
Tremor
Our data indicating that tremor was present in 63.0% of all participants with isolated focal or segmental CD are consistent with prior reports.5, 7 Prevalence of tremor affecting dystonic regions varied based on onset site within the segmental dystonia group, and was highest in focal neck onset with segmental spread compared to focal onset elsewhere that later spread to neck. Furthermore, prevalence of tremor affecting regions without dystonia remains modest across all sites of onset regardless of spread. Thus, these data support prior reports that spread of dystonia more commonly occurs in those with tremor compared to those without tremor.36 Specifically, the current data indicate that spread of dystonia from the neck to other body regions is associated more with tremor affecting the dystonic part than tremor in another region of the body. Such observations may impact counseling patients regarding symptom progression and in clinical trial design. While hand tremor occurred more often in all three segmental groups compared to focal neck onset with no spread, this did not directly translate to increased prevalence of tremor affecting regions without dystonia and thus indicate a higher prevalence of dystonic hand involvement in these groups. Indeed, participants with associated arm and hand dystonia had more tremor in a dystonic region than those with upper face involvement (e.g. blepharospasm) which may account for some group differences. Regardless, these observations may explain discrepancies of tremor prevalence across various studies of dystonia that did not account for dystonia onset and spread.17, 36-38 Our data are limited to prior classification schemes of tremor affecting dystonic regions for this analysis, but future efforts with objective tremor measures may help to further distinguish relevance of tremor to dystonic phenotypes.31 The video repository in the Dystonia Coalition dataset may allow for improved objective measures for better characterizing tremor in the future.30
In a limited sample analysis, we did not observe a difference in dystonia responsiveness to alcohol across tremor groups including tremor affecting the part of the body with or without dystonia. But, both tremor groups reported higher response rate to alcohol than participants without tremor. Alcohol responsiveness may reflect participants that have dystonia and an action tremor in parts of the body not affected by dystonia.
Response of dystonia to alcohol
There are few prior reports involving a small number of participants documenting a response of CD to alcohol that include direct observation of improvement following intravenous alcohol infusion39, 40 and survey results demonstrating 56.5% of patients with laryngeal dystonia self-reported improvement following alcohol (independent of the presence of tremor).40 Current data support a smaller percentage of individuals who report benefit from alcohol with CD, but there may be some bias as only 51% of all participants responded with a known effect (i.e. the remaining reported “unknown”) and recall of tremor response to alcohol shares additional reliability issues. Despite the observation that both groups with tremor reported higher response rate of dystonia to alcohol, future studies are thus necessary to objectively quantify the relationship of alcohol responsiveness in CD while considering onset site.
Study limitations
All exam data were collected prospectively, but the retrospective cross-sectional nature of historical data points may contribute to recall bias in participant recollection of dystonia onset, response of dystonia to alcohol or presence of sensory trick. Additionally, the population readily available for study may not reflect subcategories of dystonia that lack response to botulinum toxin since recruitment at our center may be biased to those patients that return for chemodenervation. Similarly, those with mild disease that are either under recognized or not seeking treatment will less likely attend one of our recruiting centers. With regards to botulinum toxin administration, examinations were performed at least two months after botulinum toxin injection, but data regarding the distribution of this variable are not available for many of the participants and thus could confound presence of tremor in a dystonic region and/or severity of neck dystonia. It also remains possible that the relative extent of cervical dystonia may independently influence comparability between and within groups (i.e. the neck is more affected in focal cervical dystonia than segmental dystonia, partially reflected by global dystonia rating scale and Burke-Fahn-Marsden scores in table 2). Validated interval severity rating scales to objectively compare severity of dystonia across body regions remain elusive, and future efforts should continue to develop such scales. Finally, due to the exploratory nature of analyses, strict multiple comparisons were not addressed in statistical analyses across groups that may produce additional false positive statistical errors.
Conclusions
Data from the largest standardized multi-center collection of CD data demonstrate modest but significant differences in clinical characteristics across subgroups based on onset site and spread. The differences in presence of sensory trick, response of dystonia to alcohol, and tremor affecting dystonic regions may represent distinct clinical entities for which future studies need to consider for proper experimental design and implementation. Consideration of such features may be necessary for accurate description, pathophysiologic categorization, prognosis, treatment, or modification of disease progression in those with idiopathic isolated CD. The Dystonia Coalition clinical database provides a means to collect prospective data that will allow improved data analyses along these lines.
Supplementary Material
Acknowledgments
Funding sources for study: Dystonia Coalition (U54NS065701 & U54TR001456), a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. The Dystonia Coalition is funded through collaboration between NCATS and the National Institute of Neurological Diseases and Stroke (NINDS). The study was additionally supported by grants from the NIH NINDS (NS065701, NS058714, NS41509, NS075321), Murphy Fund, American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University; Greater St. Louis Chapter of the APDA, McDonnell Center for Higher Brain Function; and the Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and the Parkinson Disease Research Fund).
Financial Disclosures of all Authors for the Past Year
Dr. Norris received grant support from the NIH/NINDS (R01NS058714, PI: Perlmutter) and travel honoraria from Medtronic, Inc. H. A. Jinnah received grant support from the NIH, the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, Merz Inc, and Ipsen Inc., served as consultant for Psyadon Pharmaceuticals and Medtronic, Inc., and was on scientific advisory boards for Cure Dystonia Now, the Dystonia Medical Research Foundation, Lesch-Nyhan Action France, the Lesch-Nyhan Syndrome Children's Research Foundation and Tyler's Hope for a Cure. Dr. Espay consulted for Abbvie, Teva, Impax, Merz, Cynapsus, Lundbeck and USWorldMeds, served on scientific advisory boards for Abbvie, TEVA, Impax, Merz, Acadia, Cynapsus, Lundbeck and US WorldMeds, received honoraria from Abbvie, USWorldMeds, Lundbeck, and Acadia, received grants from the NIH (1K23MH092735), Great Lakes Neurotechnologies, and Michael J Fox Foundation, and received royalties with Lippincott Williams & Wilkens and Cambridge University Press. Dr. Klein consulted as medical advisor to Centogene and received honoraria for speaking from Biogen Idec, Wellcome Trust Review Board, was on the scientific advisory board of the Else Kroener Fresenius Foundation, received grants from The Hermann and Lilly Schilling Foundation, German Research Foundation, BMBF, the European Community and University of Luebeck, and received royalties from the Oxford University Press. Dr. Brüggemann received a grant for the Collaborative Center for X-linked dystonia-parkinsonism and received additional research support from Zambon, Medtronic, and St. Judes Medical. Dr. Barbano owned stock in Visual Dx, received honoraria from International Toxin Association, Neurology Clinical Practice, received grants from Biotie and Vaccinex, and provided expert testimony for Medical Legal. Dr. Malaty received honoraria for speaking for the National Parkinson Foundation and Tourette Association of America and received institutional funding from the National Parkinson Foundation and Tourette Association of America Center of excellence awards as well as research funding from Abbview, Auspex, Biotie, Merz, Neurocrine, Pfizer. Dr. Rodriguez held stock in Acadia Pharmaceuticals, Noravax, Neuroderm, and Neurocrine. He consulted for Abbvie and was on scientific boards for Abbvie, Cynapsus and Teva, received honoraria from Abbvie, Allergan, and CME Meeting, had grants from Abbview, Auspex, Ipsen, and Merz and gave expert testimony for McCumber Daniels, USAttorney's Office. Dr. Vidailhet was on scientific advisory boards and consulted for Merz Pharmaceuticals and Medtronic. She received grants from the following patient associations: APTES (tremor) and AMADYS (dystonia). Dr. Roze is in scientific advisory boards for Orkyn, Arguettant, Ultragenix, and Merz pharmaceuticals. He received honoraria from Orkyn, Aguettant, and Merz Pharmaceuticals. He had research grants from AP-HP, INSERM, CNRS, Orkyn, Aguettant, IP sante, Merz Pharmaceuticals, UltraGenix, UCB pharmaceuticals, and received travel grants from the movement disorder society, dystonia coaltion, dystonia medical research foundation, Orkyn, Aguettant, Abbvie, and Sanofi-Genzyme. Dr. Reich received grants from the NIH (NINDS), royalties from Informa and is a reviewer for UpToDate. Dr. Berman received grant support from NIH/NCATS Colorado CTSI (KL2 TR001080), The Dana Foundation, NIH/NINDS R01 NS074343 (PI: Schenkman), NIH/NIMH R01 MH102224 (PI: Tregellas), NIH/NIDDK R01 DK103691 (PI: Tregallas). Dr. LeDoux consulted for Mayo Clinic Jacksonville, served on the scientific advisory boards for the National Spasmodic Torticollis Association, Spastic Paraplegia Foundation, received honoraria from Lundbeck. He had grants from NIH, Benign Essential Blepharospasm Ressearch Foundation, Omeros, Auspex, Teva, CHDI, US WorldMeds, and Accorda, received compensation for expert testimony for Starnes Davis Florie, and received royalties from Elsevier. Dr. Pirio Richardson received grant support (NCRR/NCATS KL2 1TR001448-01) and royalties from Springer. Dr. Agerwal's spouse holds stock in Exilexis, Pharmaathens, Atossa Genetics, Pain Therapeutics, Antares, MEI Pharma, Marina Biotech, RxiPharmaceuticals. Dr. Agarwal consulted for Teva, Cynapsus, US world meds, UCB, Lundbeck, and Impax, served on scientific advisory borads for Teva, Cynapsus, US world meds, UCB, Lundbeck, and Impax, received honoraria from Teva, Lundbeck, US world meds, and Imprax and has a grant funded by Astellas. Dr. Mari had grants from the NIH, NPF, MJFF, Avid, P-Cori and Abbvie. He is co-founder of NeuroTechno, Inc (MD). Dr. Ondo served on scientific advisory boards for ACADIA, USWorldMeds, Lundbeck, received honoraria as a speaker for Lundbeck, Avanir, Xenoport, and TEVA, and received grants from Tremor Research Group, Huntington Study Group, Lundbeck, and Cynapsus. Dr. Shih was compsnesated by the Tremor Research Group as a video rater for a clinical trial sponsored by Insightec, Inc. Dr. Fox consulted for Lundbeck and Orion and received honoraria from the American Academy of Neurology and Movement Disorders Society. She had grants from the NIH (Dystonia Coalition) and MJFF, contracts with Avanir, Kyowa, and Cynapsus and received royalties from Elseiver and Oxford University Press. Dr. Testa served on the scientific advisory boards for Lundbeck Pharmaceuticals (all honoraria was donated to Medical College of Virginia Foundation), received honoraria from MedLink Neurology (for updates to a comprehensive review), and she is co-PI on clinical trials funded by the Huntington Study Group and Auspex Pharmaceuticals (a wholly owned subsidiary of Teva Pharmaceuticals), and is the site PI for Enroll-HD (sponsored by CHDI Foundation), PRIDE-HD and Open Pride (sponsored by Teva Pharmaceuticals). Dr. Berardelli had grants from Allergan and Chiesi. Dr. Fahab consulted for Cala Health, received honoraria from Teva, grants from Medtronic and royalties from Springer Publishing. Dr. Troung had grants from Auspex Pharmaceuticals Inc., Abbvie Inc., Ipsen Pharmaceuticals, Merz Pharmaceuticals GmbH, NINDS, Adamas Pharmaceuticals, GE Healthcare, Kyowa Hakko Kirin Pharma Inc., Impax Pharmaceuticals Inc., Pfizer Inc., Osmotica Pharmaceuticals, Civitas Therapeutics Inc., Cynapsus Therapeutics Inc., Revance Therapeutics Inc., Allergan Pharmaceuticals, NIH/NIND/ORDR. He has consulted for for Ipsen Pharmaceuticals and Merz Pharmaceuticals GmbH. Dr. Nahab consulted for Cala Health, received honoraria from Teva, grants from Medtronic, and royalties from Springer Publishing. Dr. Xie consulted for CVS/Caremark, received honoraria from the Michael J Fox Foundation and Weston Brain Institute and grants from Michael J Fox Foundation, NIH, GE Healthcare, and University of Chicago. Dr. Hallett received honoraria from Elsevier for editing an on-line neurology news service and had grants from UniQure for a clinical trial of AAV2-GDNF for Parkinson Disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins. He maintained intellectual property rights for US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil) and received royalties from Brainsway for US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for the same (H-coil). Ms. Rosen received salary support via the Dystonia Coalition, part of the RDCRN, funded by NCATS and NINDS grants U54NS065701 and U54TR001456 and by and Huntington Disease Society of America Center of Excellence grant. Dr. Perlmutter has received fees for medical legal consultations. He serves (unpaid) on the DMRF and APDA scientific advisor boards and received honoraria from University of Michigan, St. Louis University, Parkinson Disease Foundation, Harvard University, Columbia University, and the Indiana Neurological Society. He received research funding from the NIH (NINDS, NCATS), APDA, Greater St. Louis Chapter of the APDA; HDSA. Dr. Jinnah is PI for the Dystonia Coalition which receives the majority of support through NIH grant TR001456 from the ORDR, NCATS, and NS065701 from the NINDS. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The European Dystonia Federation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association).
Footnotes
Financial Disclosures: None
Conflict of Interest: None
Author Contributions
Scott Norris performed all statistical analyses, wrote the first draft and revised the final version critically for important intellectual content. Alberto Espay contributed to generation of figures. Joel Perlmutter and H. A. Jinnah conceived the Dystonia Coalition and managed activities and supervised clinical data collection for this study. All authors contributed to data collection, data analysis, drafting, and/or revising the manuscript critically for important intellectual content, and approved the final version of the manuscript. All collaborators in the Dystonia Coalition contributed to data collection and site investigators assisted with data analysis.
Contributors:
Anja Pogarcic (Washington University in St. Louis, Site Coordinator); Jaya Mishra, Ben Wissel (University of Cincinnati, Site Coordinators); Sylwia Dankert, Johanna Junker, Anne Weibach (University of Luebeck, Site Coordinators); Michael T. Bull (University of Rochester, Site Coordinator); Kyle Rizer, Amanda Elers (University of Florida, Site Coordinators); Cecilia Bonnet, MD (Hôpital Universitaire Pitié-Salpêtrière; Site Investigator); Marta Ruiz PhD, Bertrand Degos, and Jean Michel Mayer (Hôpital Universitaire Pitié-Salpêtrière, Site Coordinators); Katherine Holmes (University of Maryland, Site Coordinator); Erika Shelton (University of Colorado - Denver, Site Coordinator); Misty M. Thompson PhD (University of Tennessee, Site Coordinator); Ashley Wegele (University of New Mexico, Site Coordinator); Carey Gonzales (Booth Gardner Parkinson's Care Center, Site Coordinator); Becky Dunlop (Johns Hopkins University, Site Coordinator); Chia Arif (University of Texas, Site Coordinator); Christine Ashton (Beth Israel Deaconess Medical Center, Site Coordinator); Brandon Rothberg (Toronto Western Hospital, Site Coordinator); Gina Gerrazzano (University of Rome, Site Coordinator); Virginia Norris, Daniel Demers (Virginia Commonwealth University, Site Coordinators); Victor SC Fung PhD, FRACP, Jane M Griffith (Westmead Hospital, Site Coordinators); Trong-Tuong Binh Nguyen (The Parkinson and Movement Disorder Institute, Site Coordinators); Lissette Moreno (University of California, San Diego, Site Coordinator); Joan Young (University of Chicago, Site Coordinator); Vesper Fe Marie, Jung Park, Karin Mente, Hyunjoo Cho, Elaine Considine (National Institute of Health, Site Coordinators); Cynthia Comella, MD (Rush University Site Investigator); Tracy Waliczek, (Rush University, Site Coordinator); Joseph Jankovic, MD and Laura Marsh, MD (Baylor College of Medicine, Site Investigators); Farah Ismail (Baylor College of Medicine, Site Coordinator); Natvidad Stover (University of Alabama at Birmingham, Site Investigator); Ashlee Brooke Rawlins (University of Alabama at Birmingham, Site Coordinator); Lawrence Severt, MD (Beth Israel Medical Center, Site Investigator); Emily Muller and (Beth Isreal Medical Center, Site Coordinator); Sylvian Chouinard (Centre hospitalier de l'Université de Montréal, Site Investigator); Monica Beland (Centre hospitalier de l'Université de Montréal,, Site Coordinator), Alison Brashear, MD (Wake Forest School of Medicine, Site Investigator); Charlotte Miller (Wake Forest Health Sciences, Site Coordinator); Julie Leegwater-Kim, MD (Lashey Clinic, Site Investigator); Caitlin Scopa (Lahey Clinic, Site Coordinator); Tanya Harlow, MD (Sanford Health-Fargo, Site Investigator); Stephanie Gonzales, Destini Spaeth (Sanford Health, Fargo, Site Coordinator); Oksana Suchowersky, MD (University of Alberta, Site Investigator); Paul McCann (University of Alberta, Site Coordinator); Charles Alder, MD (Mayo Clinic Arizona, Site Investigator); Amy Duffy (Mayo Clinic Arizona, Site Coordinator); Stephen Grill, MD, PhD (Parkinson's and Movement Disorders Center of Maryland, Site Investigator); Erica Stacy (Parkinson and Movement Disorder Center of Maryland, Site Coordinator); Joel Blumin, MD (Medical College of Wisconsin, Site Investigator); Lynn Wheeler (Medical College of Wisconsin, Site Coordinator); Ergun Uc, MD (University of Iowa, Site Investigator); Jeri Sieren RN (University of Iowa, Site Coordinator); Kailash Bhatia, FRCP (University College of London, Site Investigator); Bettina Balint (University College of London, Site Coordinator).
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jinnah HA, Berardelli A, Comella C, et al. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;28:926–943. doi: 10.1002/mds.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubio-Agusti I, Parees I, Kojovic M, et al. Tremulous cervical dystonia is likely to be familial: clinical characteristics of a large cohort. Parkinsonism Relat Disord. 2013;19:634–638. doi: 10.1016/j.parkreldis.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rondot P, Marchand MP, Dellatolas G. Spasmodic torticollis--review of 220 patients. Can J Neurol Sci. 1991;18:143–151. doi: 10.1017/s0317167100031619. [DOI] [PubMed] [Google Scholar]
- 5.Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci. 2000;27:137–142. [PubMed] [Google Scholar]
- 6.Muller J, Wissel J, Masuhr F, Ebersbach G, Wenning GK, Poewe W. Clinical characteristics of the geste antagoniste in cervical dystonia. J Neurol. 2001;248:478–482. doi: 10.1007/s004150170156. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 8.Duane DD. Spasmodic torticollis: clinical and biologic features and their implications for focal dystonia. Adv Neurol. 1988;50:473–492. [PubMed] [Google Scholar]
- 9.Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547–560. doi: 10.1093/brain/121.4.547. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 11.Abbruzzese G, Berardelli A, Girlanda P, et al. Long-term assessment of the risk of spread in primary late-onset focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:392–396. doi: 10.1136/jnnp.2007.124594. [DOI] [PubMed] [Google Scholar]
- 12.Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol. 2004;94:101–107. [PubMed] [Google Scholar]
- 13.Greene P, Kang UJ, Fahn S. Spread of symptoms in idiopathic torsion dystonia. Mov Disord. 1995;10:143–152. doi: 10.1002/mds.870100204. [DOI] [PubMed] [Google Scholar]
- 14.Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord. 2012;27:1447–1450. doi: 10.1002/mds.25088. [DOI] [PubMed] [Google Scholar]
- 15.Svetel M, Pekmezovic T, Jovic J, et al. Spread of primary dystonia in relation to initially affected region. J Neurol. 2007;254:879–883. doi: 10.1007/s00415-006-0457-8. [DOI] [PubMed] [Google Scholar]
- 16.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13:402–411. doi: 10.1002/ana.410130406. [DOI] [PubMed] [Google Scholar]
- 18.Marsden CD. Blepharospasm-oromandibular dystonia syndrome (Brueghel's syndrome). A variant of adult-onset torsion dystonia? J Neurol Neurosurg Psychiatry. 1976;39:1204–1209. doi: 10.1136/jnnp.39.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defazio G, Berardelli A, Abbruzzese G, et al. Risk factors for spread of primary adult onset blepharospasm: a multicentre investigation of the Italian movement disorders study group. J Neurol Neurosurg Psychiatry. 1999;67:613–619. doi: 10.1136/jnnp.67.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svetel M, Pekmezovic T, Tomic A, Kresojevic N, Kostic VS. The spread of primary late-onset focal dystonia in a long-term follow up study. Clin Neurol Neurosurg. 2015;132:41–43. doi: 10.1016/j.clineuro.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Marsden CD, Harrison MJ. Idiopathic torsion dystonia (dystonia musculorum deformans). A review of forty-two patients. Brain. 1974;97:793–810. doi: 10.1093/brain/97.1.793. [DOI] [PubMed] [Google Scholar]
- 22.Vemula SR, Xiao J, Zhao Y, et al. A rare sequence variant in intron 1 of THAP1 is associated with primary dystonia. Mol Genet Genomic Med. 2014;2:261–272. doi: 10.1002/mgg3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao J, Bastian RW, Perlmutter JS, et al. Novel human pathological mutations. Gene symbol: THAP1. Disease: dystonia 6. Hum Genet. 2010;127:470. [PubMed] [Google Scholar]
- 24.Xiao J, Bastian RW, Perlmutter JS, et al. High-throughput mutational analysis of TOR1A in primary dystonia. BMC Med Genet. 2009;10:24. doi: 10.1186/1471-2350-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao J, Uitti RJ, Zhao Y, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDoux MS, Vemula SR, Xiao J, et al. Clinical and genetic features of cervical dystonia in a large multicenter cohort. Neurol Genet. 2016;2:e69. doi: 10.1212/NXG.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahn S, Marsden CD, Calne DB. Dystonia 2. Raven Press; New York: 1988. [Google Scholar]
- 29.Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. doi: 10.1016/S1474-4422(04)00907-X. [DOI] [PubMed] [Google Scholar]
- 30.Yan L, Hicks M, Winslow K, et al. Secured web-based video repository for multicenter studies. Parkinsonism Relat Disord. 2015;21:366–371. doi: 10.1016/j.parkreldis.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deuschl G. Dystonic tremor. Rev Neurol (Paris) 2003;159:900–905. [PubMed] [Google Scholar]
- 32.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. Wiley; New York: 2000. [Google Scholar]
- 34.Jahanshahi M, Marion MH, Marsden CD. Natural history of adult-onset idiopathic torticollis. Arch Neurol. 1990;47:548–552. doi: 10.1001/archneur.1990.00530050070014. [DOI] [PubMed] [Google Scholar]
- 35.Patel N, Hanfelt J, Marsh L, Jankovic J. Alleviating manoeuvres (sensory tricks) in cervical dystonia. J Neurol Neurosurg Psychiatry. 2014;85:882–884. doi: 10.1136/jnnp-2013-307316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defazio G, Gigante AF, Abbruzzese G, et al. Tremor in primary adult-onset dystonia: prevalence and associated clinical features. J Neurol Neurosurg Psychiatry. 2013;84:404–408. doi: 10.1136/jnnp-2012-303782. [DOI] [PubMed] [Google Scholar]
- 37.Erro R, Rubio-Agusti I, Saifee TA, et al. Rest and other types of tremor in adult-onset primary dystonia. J Neurol Neurosurg Psychiatry. 2014;85:965–968. doi: 10.1136/jnnp-2013-305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudzinska M, Krawczyk M, Wojcik-Pedziwiatr M, Szczudlik A, Wasielewska A. Tremor associated with focal and segmental dystonia. Neurol Neurochir Pol. 2013;47:223–231. doi: 10.5114/ninp.2013.35584. [DOI] [PubMed] [Google Scholar]
- 39.Biary N, Koller W. Effect of alcohol on dystonia. Neurology. 1985;35:239–243. doi: 10.1212/wnl.35.2.239. [DOI] [PubMed] [Google Scholar]
- 40.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015;262:1548–1556. doi: 10.1007/s00415-015-7751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.