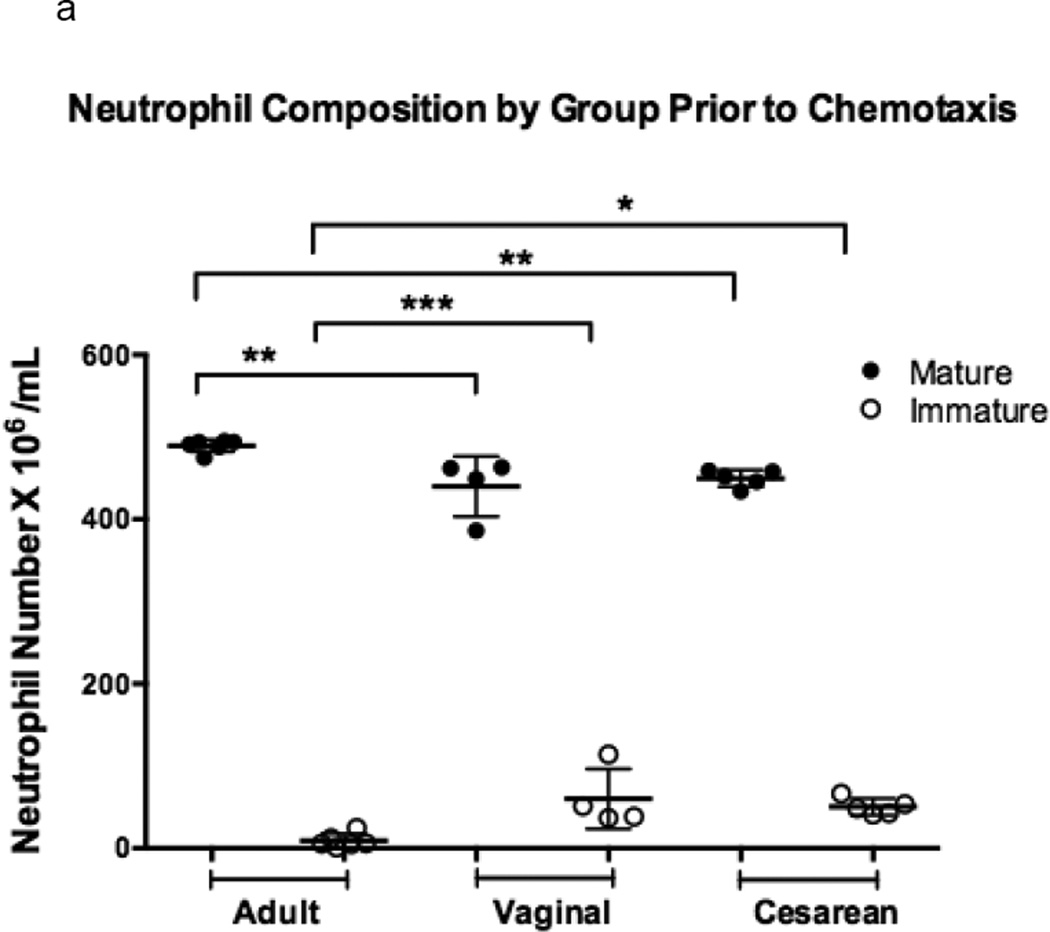
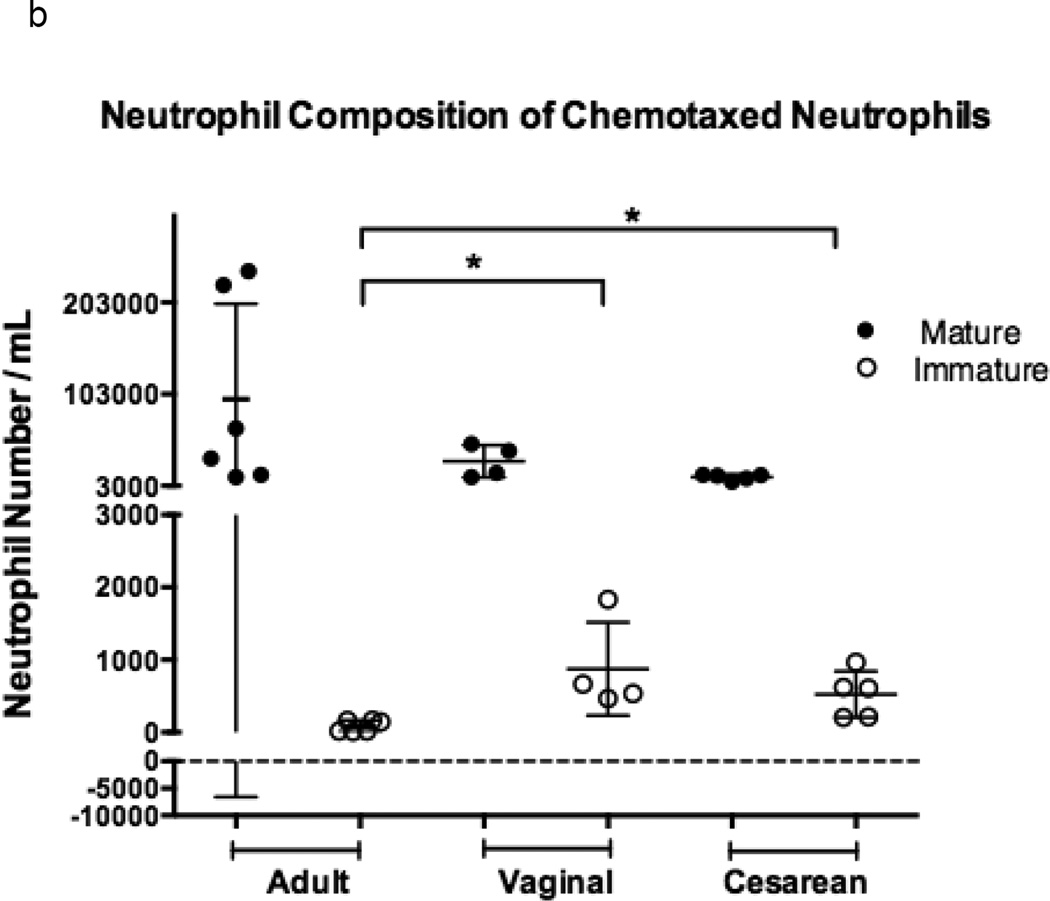
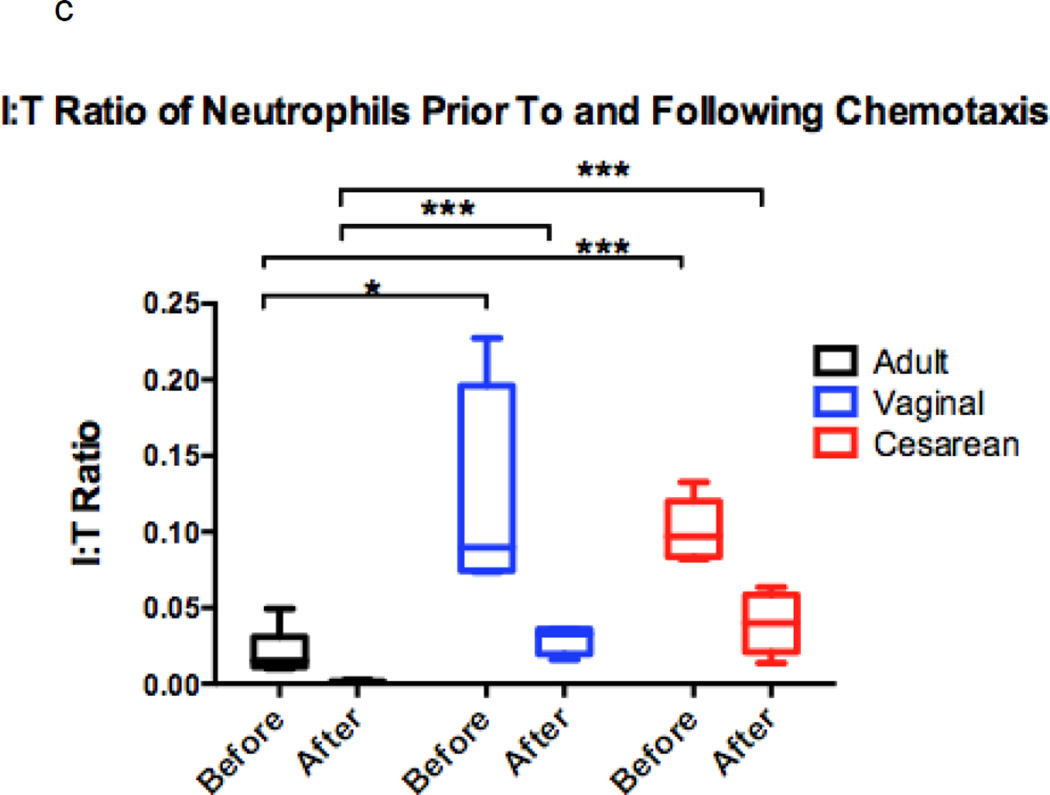
Figure 1. Comparison of chemotaxis based on neutrophil maturation between experimental groups.
Transwells containing a 12-mm diameter membrane (3 µm pores) coated with fibrinogen and using the chemoattractant, fMLP (1 × 10−7 M) or buffer alone, were used to measure chemotaxis in vaginal (n=6), cesarean (n=4,), and adult (n=5) neutrophil samples. Purified neutrophils were counted and resuspended to a concentration of 4 × 105 live cells in 200 µL Gey’s Buffer. Cells were allowed to migrate for 2 hours at 37°C and 5% CO2. Maturity of chemotaxed cells was determined by labeling of CD11b and CD16. Mature cells were defined by a population of cells high in CD11b (APC) and CD16 (PE), and consisted of segmented and banded neutrophils (closed circles). Immature neutrophils had lower levels of CD11b (APC) staining and varied levels of CD16 (PE). Metamyelocytes, promyelocytes, and myelocytes comprised the immature cell population (open circles). Neonates had a neutrophil composition consisting of a statistically higher number of immature neutrophil forms prior to (a) and (b) following chemotaxis. The total number of neutrophils completing chemotaxis was similar for all experimental groups (b). Comparison of immature to total neutrophils (I:T ratio) for each group is represented and demonstrates an increased number of immature granulocytes in neonates prior to and following chemotaxis (c). Neonates had similar findings irrespective of labor exposure (a–c). *p = 0.01, **p < 0.001, ***p< 0.0001.