Figure 1.
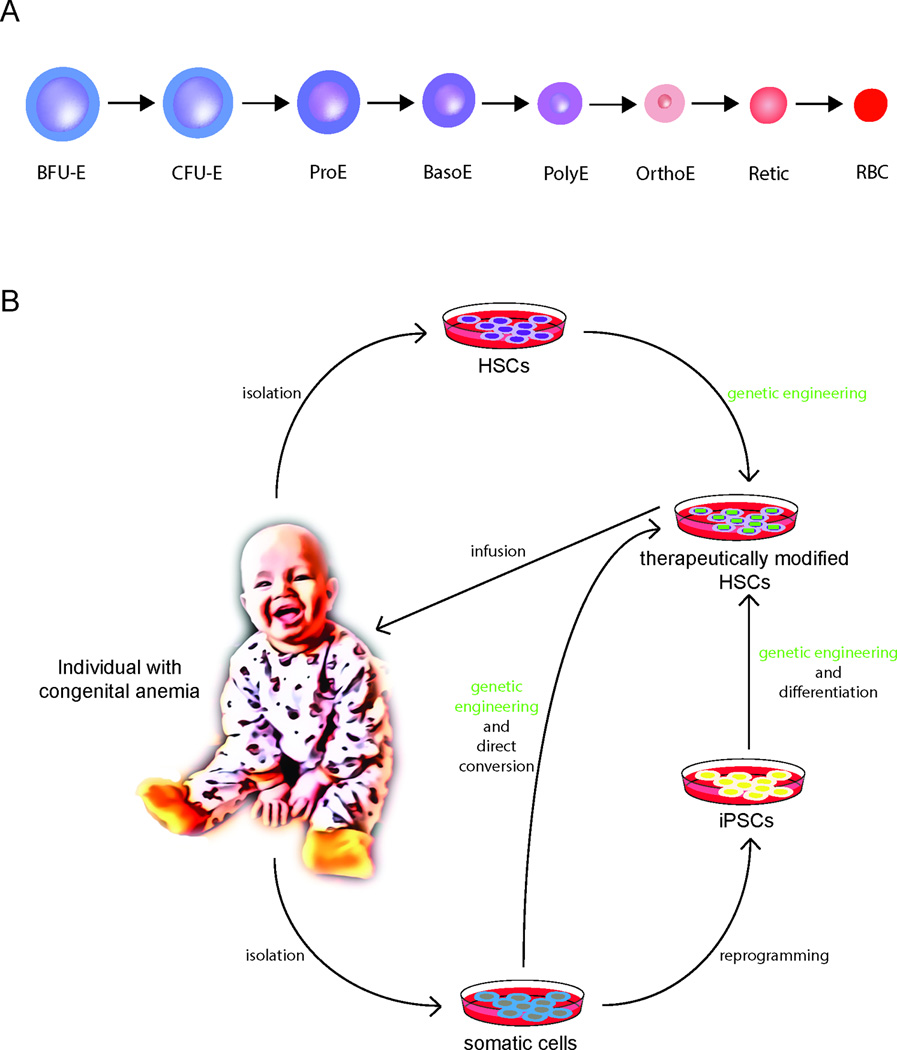
(A) A model for erythropoiesis. Early progenitor cells undergo progressive maturation through successive cellular stages that are accompanied by distinct changes in morphology. Burst forming unit – erythroid (BFU-E); colony forming unit – erythroid (CFU-E); proerythroblast (ProE); basophilic erythroblast (BasoE); polychromatic erythroblast (PolyE); orthochromatic erythroblast (OrthoE); reticulocyte (Retic); red blood cell (RBC). (B) Therapeutic avenues for the treatment of congenital anemias. Gene correction and editing of patients’ autologous cells presents a highly desired strategy to overcome limitations presented by allogeneic HSC transplantation and limited availability of HLA-matched allogeneic donors. Generally, this involves the isolation of the patient’s HSPCs from the bone marrow and subsequent exposure to gene modifying agents using viral or other transient delivery methods. Depending on the genetic entity of the disease, correction may be achieved by the introduction of a functional gene, repair of the mutated gene at its endogenous locus by homology directed repair (HDR) (e.g. the SCD mutation), or by targeting a modifier gene or its regulators known to ameliorate disease (e.g. knockdown or disruption of BCL11A in the hemoglobinopathies). The corrected cells would subsequently be re-infused into the patient to allow repopulation of the bone marrow and generation of healthy cells. Patient-specific induced pluripotent stem cells (iPSCs) derived through reprogramming of somatic cells present another possible approach. They have an unlimited propagation capacity allowing for more faithful genetic modification as they can be more extensively screened for adequate correction compared to manipulated HSPCs. Subsequently, they may be directed to differentiate into hematopoietic cells. Alternatively, a patient’s somatic cells could be directly converted into hematopoietic cells. However, in both instances the derivation of HSPCs with multi-lineage potential and engraftment capability remain key challenges to be overcome.
