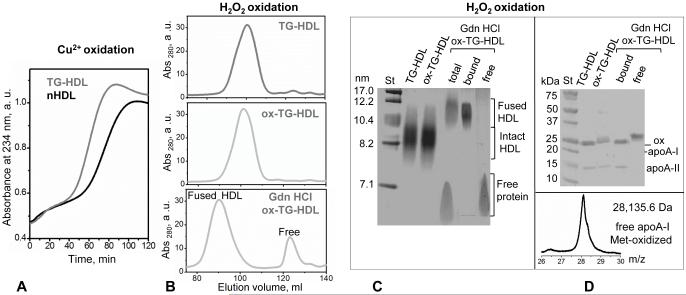
Figure 5.
TG enrichment accelerates HDL oxidation that promotes dissociation of methionine-oxidized free apoA-I. (A) Kinetics of copper-induced oxidation of nHDL and TG-HDL monitored by absorbance at 234 nm for conjugated diene formation. (B) Mild oxidation of TG-HDL by hydrogen peroxide. TG-HDL that were intact (top), oxidized (middle, marked ox-TG-HDL) or oxidized and treated with 3M Gdn HCl (bottom) were analyzed by SEC using Superdex-XK 200 column. (C) Native PAGE of the total samples and of the SEC fractions of the Gdn HCl-treated ox-TG-HDL that showed fusion and release of free protein (panel B). (D) SDS PAGE of the total samples from panel B and their SEC fractions shows that free protein released from ox-TG-HDL contains oxidized apoA-I. Molecular mass of this free protein, which was determined by MALDI TOF (D, bottom), was increased by 48 Da compared to unmodified apoA-I, suggesting oxidation of the three methionines (M86, M112 and M148).