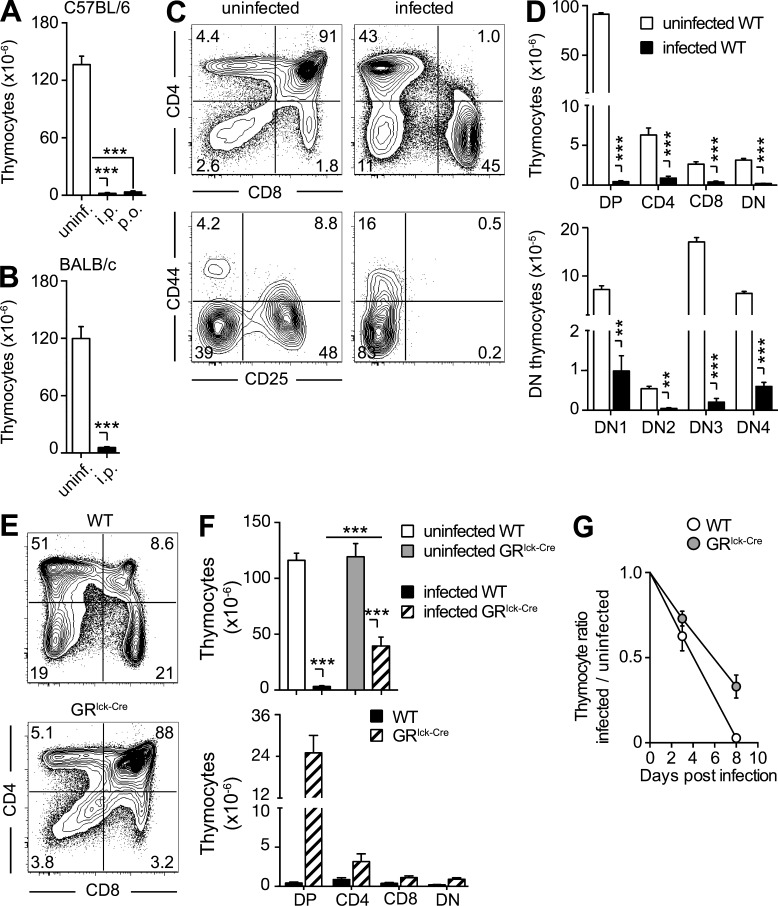
Figure 3.
The thymic atrophy induced by toxoplasma infection is mouse strain and infection route independent, but only partially GC dependent. (A and B) The severe loss of thymocytes during acute toxoplasma infection is independent of both the route of exposure and host genetic background. Bars represent the mean ± SEM of thymocytes in C57BL/6 mice (n = 6–9) uninfected or infected for 10 d by either i.p. or gavage (p.o.; A), and in uninfected or 10 d i.p. infected BALB/c mice (n = 4; B) from one representative out of two experiments performed. (C and D) All thymocyte subsets are decreased on day 10 after T. gondii infection. (C) Representative dot-plots of total (B220− NK1.1− CD11b−) thymocytes stained with anti-CD4 and anti-CD8 antibody (top) and of double negative (DN) thymocytes stained with anti-CD44 and anti-CD25 antibody (bottom). (D) Bar graph showing the mean ± SEM of DP, CD4, CD8, and double-negative (DN) thymocytes (top) or DN1-4 populations (bottom) for individual animals (n = 6) pooled from two independent infections. (E–G) Lack of GR signaling only partially rescues thymic atrophy. (E) Representative dot plots of anti-CD4– and anti-CD8–stained thymocytes from 8-d infected WT or GRlck-Cre animals, and (F) bar graphs representing the mean ± SEM of total (top) and DP, CD4, CD8, and DN (bottom) thymocytes in each group (n = 10) pooled from three independent experiments. (G) Kinetics of thymocyte loss in infected WT or GRlck-Cre mice calculated as the ratio between number of thymocytes on day 0 and days 3 or 8 after infection (n = 4–8) from two time course experiments performed. **, P < 0.01; ***, P < 0.001.