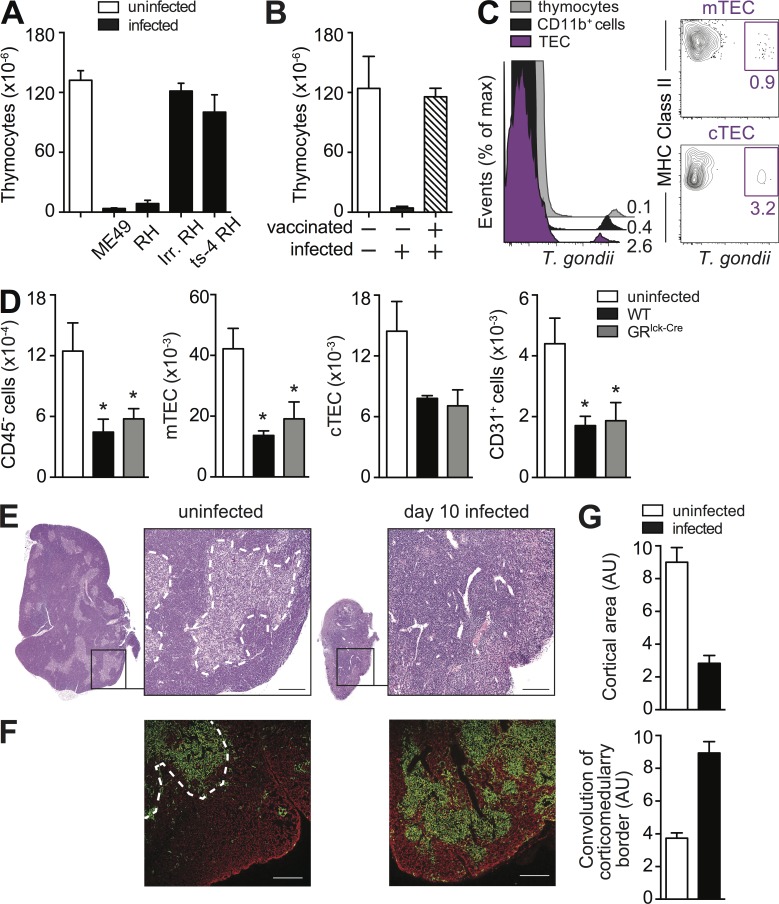
Figure 5.
The thymic atrophy induced by T. gondii requires parasite replication and is associated with epithelial cell (TEC) disruption. (A and B) Thymic atrophy is observed after i.p. challenge with either type I (RH) or type II (ME49) strains of T. gondii but not with nonreplicating live-irradiated RH or a temperature sensitive mutant (ts-4) of that strain (A) or after challenge with WT parasites in previously vaccinated hosts (B). Bars represent the mean ± SEM of thymocytes assayed on day 8 in mice (A) infected i.p. with indicated strains of parasites (n = 4) or (B) challenged with RH after vaccination with ts-4 (n = 3). Data are representative of one of two independent experiments performed. (C) Phenotype of T. gondii–infected cells in the thymus. Mice were infected with mCherry-expressing tachyzoites and on day 3 FACS analyses were performed. Overlay histograms gated on total TEC population, CD11b+ myeloid cells, and thymocytes (left) and contour-plots gated on mTEC and CTEC (right) from one of three independent experiments with at least three infected mice each. (D) Loss of TEC during acute toxoplasma infection. TEC cells were isolated from uninfected WT mice, and day 8 infected WT and GRlck-Cre animals and were analyzed by FACS for mTEC (UEA+ I-Ab+), cTEC (Ly51+ I-Ab+), and CD31+cells. Bars represent the mean numbers (± SEM) of cTEC, mTEC, and CD31+ endothelial cells calculated from analyses performed on individual animals (n = 6–8) pooled from two independent experiments. (E) Cross section of thymus lobe from naive or day 8 infected WT mice stained with hematoxylin and eosin with indicated portion magnified 10×. Bar, 100 µm. (F) Image of a thymic section from uninfected and day 8 infected WT mice after immunostaining with anti-keratin 5 (mTEC) and anti-keratin 8 (cTEC) Antibodies, shown in green and red, respectively. Bar, 150 µm. (G) Quantitative representation of cortical area and convolution of corticomedulary border in thymii from uninfected and infected mice pooled from two experiments (n = 6). *, P < 0.05.