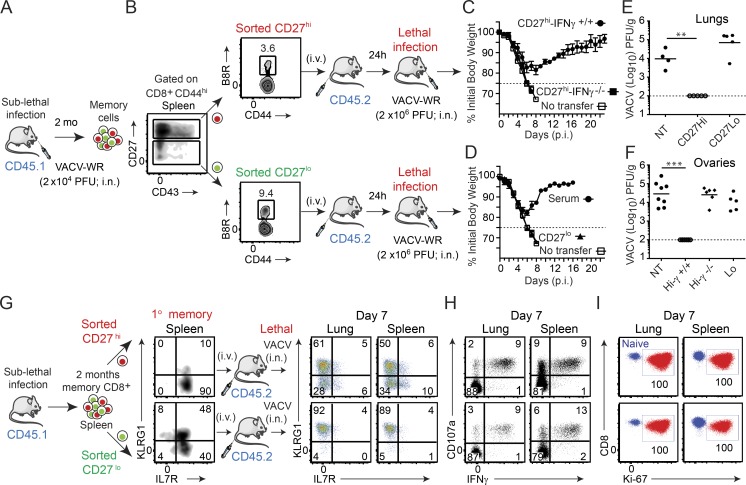
Figure 1.
CD27hi memory CD8 T cells mediate optimal protection against lethal respiratory VACV-WR infection. (A) Cohorts of WT donor mice (B6.SJL; CD45.1+) were infected i.n. with VACV-WR (1.5 × 104 PFU/mouse). (B) Spleens were harvested 60 d later. Cells were enriched for CD8+ cells on negative selection columns and sorted into CD8+CD44hiCD27hi and CD8+CD44hiCD27lo subsets by FACS. The sorted CD27hi (C) and CD27lo (D) cells were then adjusted such that the number of B8R20–27/H-2Kb cells was equivalent (∼30,000 tetramer+ cells) and i.v. transferred into naive (CD45.2+) recipient mice. (D) Some groups received 250 µl VACV-immune serum i.p. 1 d after transfer, recipient mice were infected i.n. with a lethal inoculum of VACV-WR (2 × 106 PFU). Naive mice that did not receive memory cells were used as controls (No transfer [NT]). Animals were weighed daily and euthanized if weight loss was >25% of initial body weight for two consecutive days. (C and D) Mean percent of initial body weight is shown. On day 7 after challenge lung (E) and ovaries (F) from individual mice that survived the infection were collected and virus titers were determined by plaque assay as described in Materials and methods. Dotted line indicates limit of detection for the plaque assay. Each symbol represents one mouse. (G–I) Mice that received CD8+CD44hi CD27hi and CD27lo memory T cells were analyzed on day 7 after infection. Lung and spleen mononuclear cells were harvested and stained for CD8α, CD44, KLRG1, IL-7Rα, CD45.1, B8R20-27/Kb tetramer, intranuclear Ki-67, or stimulated with B8R peptide and subsequently stained for CD107a and intracellular IFN-γ. Representative plots for KLRG1/IL-7Rα (G), CD107a/IFN-γ (H), and Ki-67 (I) staining gated on CD8+CD44hiCD45.1+B8R20-27/Kb-tetramer+ (G and I) and CD8+CD44hiCD45.1+ (H) cells are shown. Numbers indicate percentages of positive cells within the gated population. Quadrant settings were based on controls, after gating on CD44lo cells in the same host for phenotypic analysis or using infected cells that were not stimulated with peptide and uninfected cells stimulated with B8R peptide for functional analysis (not depicted). Data show one representative experiment of at least four with same results with at least four mice per experimental group. Error bars, SEM (C and D). **, P < 0.01; ***, P < 0.001 (two-tailed Student’s t test).