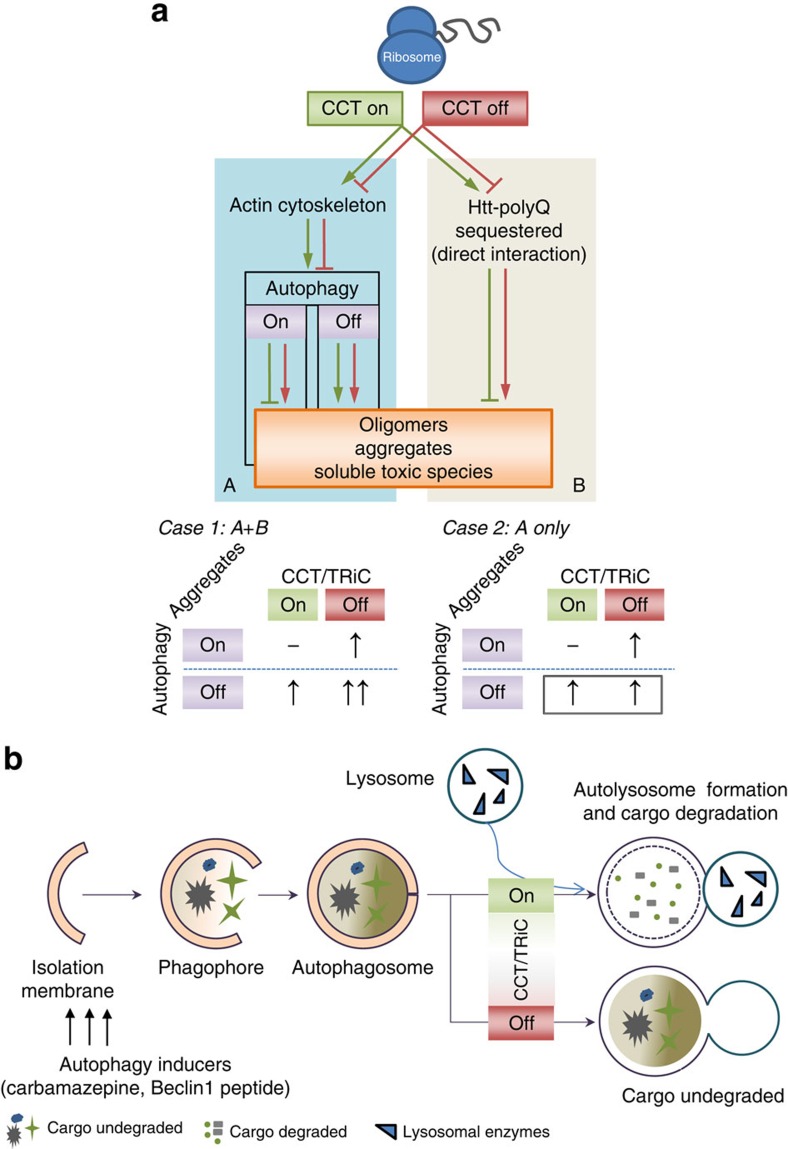
Figure 9. Model for the autophagy-dependent oligomerization/aggregation of mutant htt in CCT-depleted cells.
(a) Accumulation of oligomers, soluble toxic species and aggregates of mutant htt under CCT depletion may either be due to case 1: autophagy defects combined with reduced direct CCT interaction/sequestration of the mutant proteins, or case 2: autophagy inhibition only. In autophagy-competent cells, CCT depletion increases the mutant htt oligomerization and aggregation, compared with control. However in autophagy-incompetent cells, CCT depletion does not have additional effects on aggregate-prone protein accumulation, compared with control. This suggests that autophagy plays the major role in mutant htt aggregation in cell and in vivo models, and that any direct role of CCT in oligomerization of proteins like EGFP-HTT(Q74) is undetectable using the types of assays we have used. Since the readouts we have employed are not saturated, we conclude that proposed direct chaperoning effects of CCT on the oligomerization/aggregation of Q74 and related proteins is at best very small, compared with the autophagy effect of CCT compromise. (b) Schematic overview of autophagy-lysosome pathway defects under CCT depletion and subsequent impact on mutant proteins aggregation. Since the final step in autophagy–lysosome pathway is severely compromised, autophagy inducers acting at the level of autophagosome biogenesis (carbamazepine or Beclin1 peptide) are not able to impact on the aggregation phenotype.