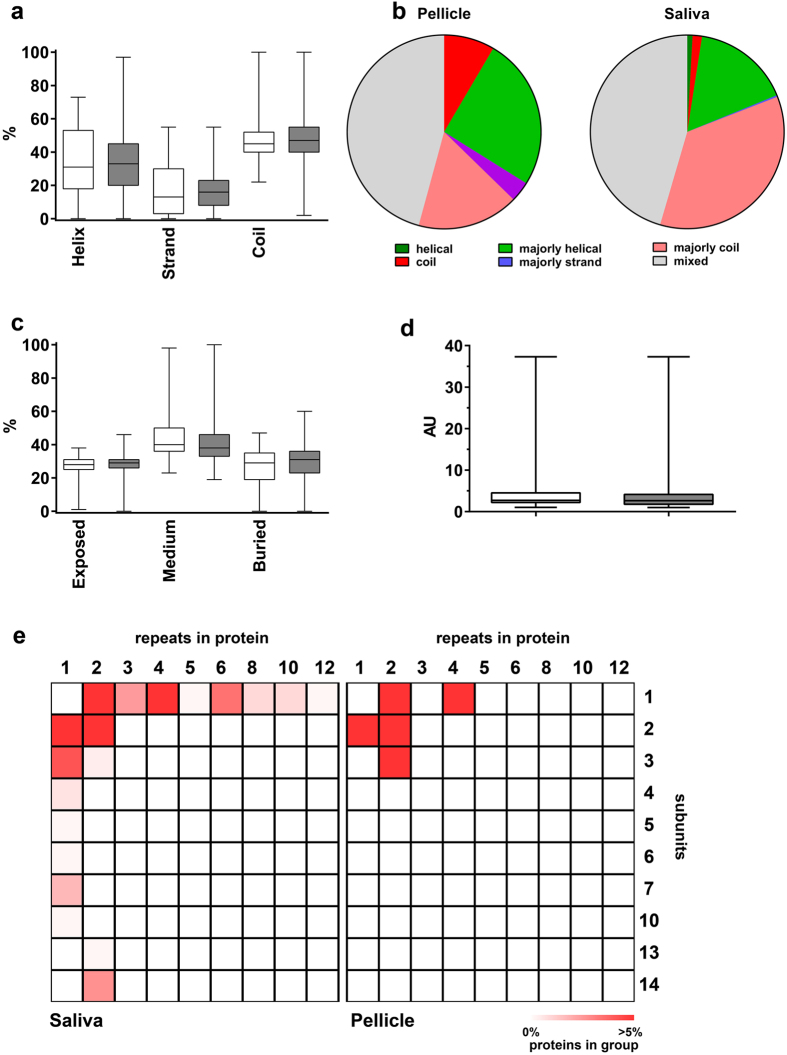
Figure 4. Predicted secondary structure motives, solvent surface access, molecular shape and organization of pellicle and salivary proteins.
(a) Typical structures like alpha helices, beta sheets and coils were not predicted significantly different for both proteomes (pellicle: white; saliva: grey). (b) The pellicle included more proteins with an almost coiled structure, but no proteins consisting only alpha helices. Overall, salivary proteins were more organized. Categories: “helical/strand/coil” = >90% of amino acids are predicted to be organized in this motif; “majorly helical/majorly strand/majorly coil” = 50–90% are predicted to be included in these motives; “mixed” = <50% of amino acids are belonging to one type of secondary structure motif. (c) The frequency of exposed or buried amino acids did also not differ significantly (pellicle: white; saliva: grey). (d) Both proteomes (pellicle: white; saliva: grey) had comparable distribution of molecular shapes. A score of 1 indicates a perfectly spherical shape, higher scores indicate more stretched shapes. AU = arbitrary units (e) Quaternary structure organization was more complex for salivary proteins. The pellicle included more proteins with fewer repeats and subunits. Salivary proteins are more often organized in larger complexes of up to 14 subunits and 12 repeats. Statistical comparison of proteomes was performed using Mann-Whitney-U test.