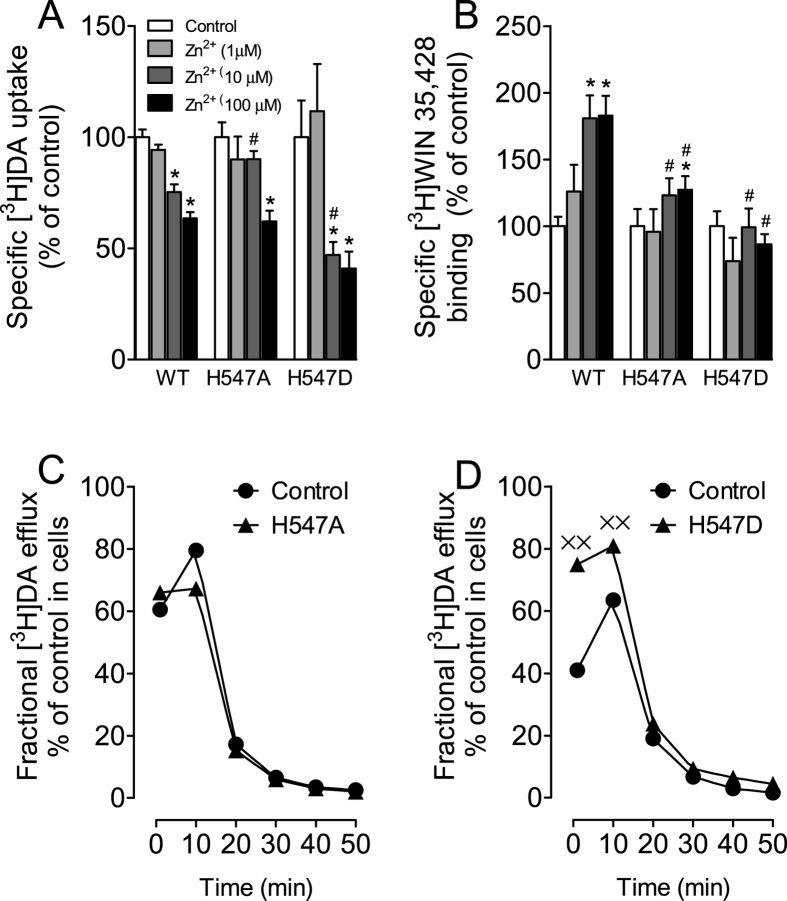
Figure 5. Effects of H547A and H547D mutants on transporter conformational transitions.
Mutations of His547 affect zinc regulation of [3H]DA uptake (A) and [3H]WIN 35,428 binding (B). PC12 cells transfected with WT hDAT (WT), H547A-hDAT (H547A) and H547D-hDAT (H547D) were incubated with KRH buffer alone (control) or ZnCl2 (1, 10, 100 μM, final concentration) followed by [3H]DA uptake or [3H]WIN 35,428 binding (n = 5–7). The histogram shows [3H]DA uptake and [3H]WIN 35,428 binding expressed as mean ± S.E.M. of the respective controls set to 100% for the mutant. *p < 0.05 compared to control. #p < 0.05 compared to WT hDAT with ZnCl2. Functional DA efflux of DA properties of H547A-hDAT (C) and H547D-hDAT (D) with their respective WT hDAT control. PC12 cells transfected with WT hDAT or mutants were preincubated with KRH buffer containing [3H]DA (0.05 μM, final concentration) at room temperature for 20 min. After incubation, cells were washed and incubated with fresh buffer as indicated time points. Subsequently, the buffer was removed from cells, and radioactivity in the buffer and remaining in the cells was counted. Each fractional efflux of [3H]DA in WT hDAT (WT) or mutants was expressed as percentage of total [3H]DA in the cells at the start of the experiment. Fractional [3H]DA efflux levels at 1, 10, 20, 30, 40 and 50 min are expressed as the percentage of total [3H]DA with preloading with 0.05 μM (WT hDAT: 13743 ± 3050 dpm, H547A-hDAT: 14464 ± 2547 dpm and H547D-hDAT: 1891 ± 428 dpm) presented in the cells at the start of the experiment (n = 4). ××p < 0.05 compared to WT hDAT (Bonferroni t-test).