Figure 1.
Interaction of MYB75 with MPK4 Is Dependent on MPK4 Kinase Activity.
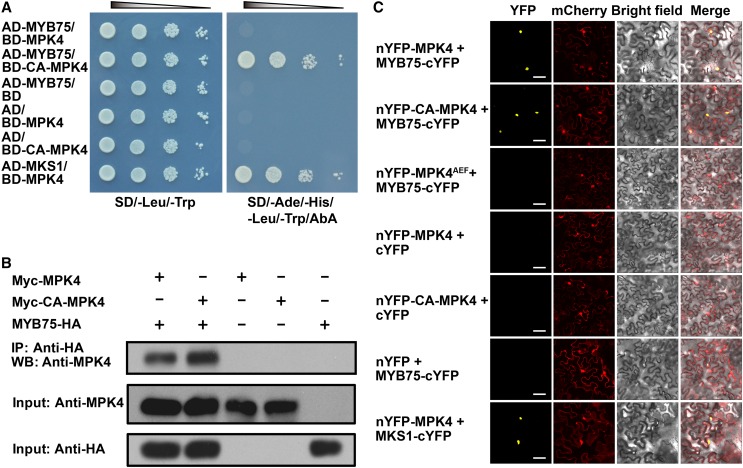
(A) Constitutively active MPK4 (CA-MPK4), but not native MPK4, interacts with MYB75 in yeast. Serial dilutions of transformed yeast cells were spotted on the indicated amino acid dropout agar plates. Cotransformed MPK4 and MKS1 were used as a positive control. AD, GAL4 activation domain; BD, GAL4 DNA binding domain. SD, synthetically defied medium; AbA, Aureobasidin A.
(B) Coimmunoprecipitation of MPK4 and CA-MPK4 with MYB75. MYB75-HA was coexpressed with Myc-MPK4 and Myc-CA-MPK4 in N. benthamiana leaves by agroinfiltration. Leaf lysates were immunoprecipitated with anti-HA beads and stained with anti-MPK4. One-twentieth of the input proteins was stained with the same antibodies as loading control. “+” and “−” denote presence and absence of the protein in each sample, respectively.
(C) A BiFC assay in N. benthamiana leaves showing that MYB75 interacts with MPK4 and CA-MPK4, but not with kinase-dead MPK4AEF. mCherry was coexpressed as a transformation control. Yellow indicates a positive interaction signal, and red indicates signals from mCherry. Bars = 50 μm.