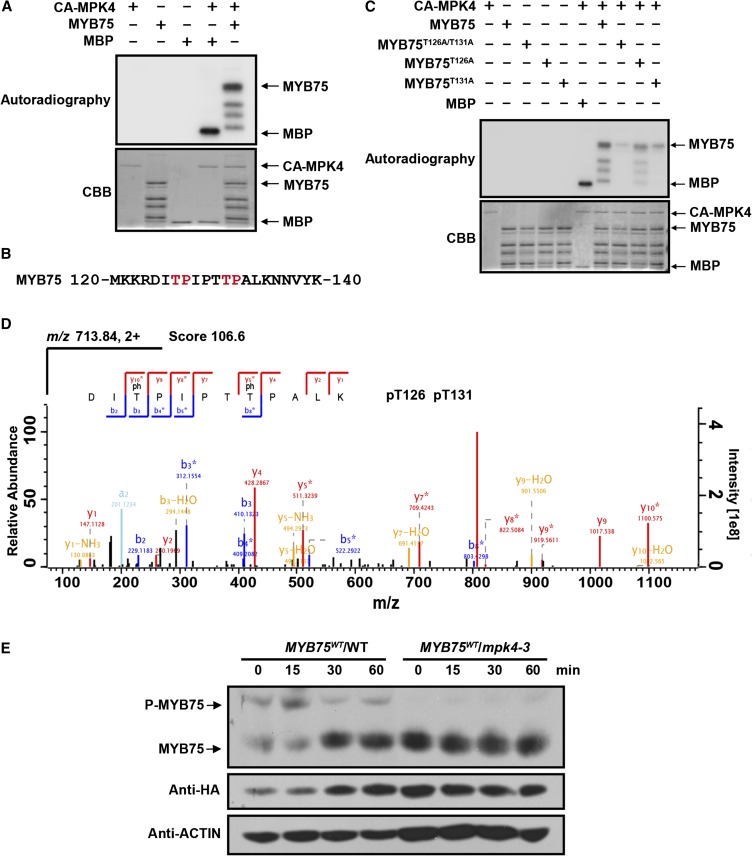
Figure 5.
MPK4 Phosphorylates MYB75.
(A) Phosphorylation of recombinant MYB75 by CA-MPK4. CA-MPK4 was used to phosphorylate purified His-tagged MYB75. Side-by-side control reactions using myelin basic protein (MBP) as a substrate confirmed the kinase activity of CA-MPK4. Phosphorylated MYB75 was visualized by autoradiography after gel electrophoresis.
(B) Putative MAPK phosphorylation sites (marked in red) in the MYB75 protein sequence.
(C) Mutation of the putative MAPK phosphorylation sites greatly reduces the phosphorylation of MYB75 by CA-MPK4. Recombinant MYB75, MYB75T126A, MYB75T131A, and MYB75T126A/T131A were incubated with CA-MPK4. Phosphorylated MYB75 was visualized by autoradiography after gel electrophoresis.
(D) LC-MS/MS analysis showing that MYB75 Thr-126 and Thr-131 are phosphorylated. The sequence of the doubly charged peptide ion at m/z 713.84, score 106.6, matches DIpTPIPTpTPALK of MYB75. “b” and “y” denote peptide fragment ions retaining charges at the N and C terminus, respectively. The subscript numbers indicate positions in the identified peptide. pT indicates phosphorylated Thr.
(E) In vivo phosphorylation of MYB75. Twelve-day-old seedlings of 35S:MYB75WT/WT and 35S:MYB75WT/mpk4-3 were exposed to high light for the indicated times. Protein extracts were separated on SDS-PAGE gels with Phos-tag reagent, and MYB75 was detected with anti-HA antibody. Due to the low MYB75 levels in mpk4-3, double the amount of total protein extract was loaded for the mpk4-3 samples than from the wild type for comparative purposes.