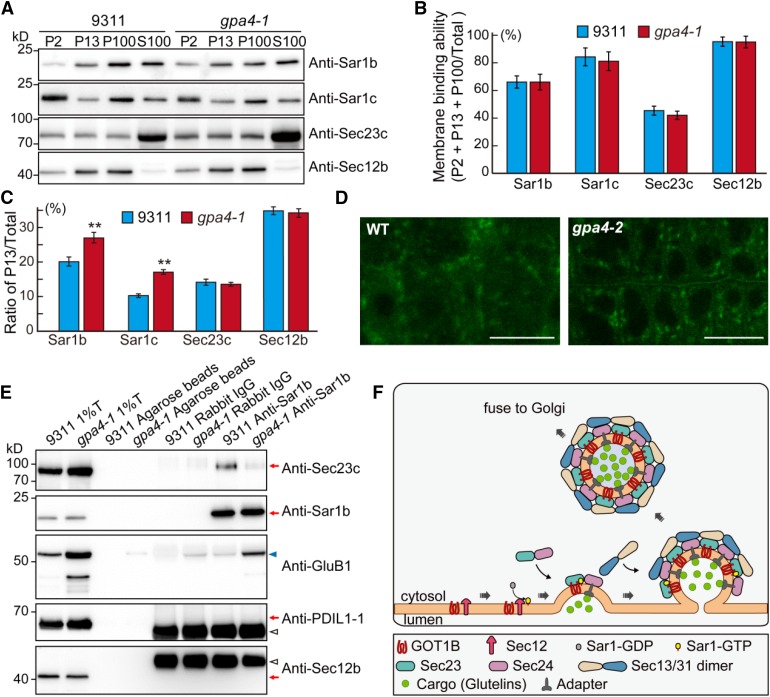
Figure 8.
Mutation of GOT1B Affects the Distribution of COPII Coat Components and ERESs.
(A) Immunoblot analysis showing the distribution of COPII coat components in the various membrane fractions in the wild type and gpa4-1. P2, pellet obtained following the centrifugation at 2000g; P13, pellet obtained following the centrifugation at 13,000g; P100, pellet obtained following centrifugation at 100,000g; S100, supernatant obtained following centrifugation at 100,000g.
(B) Quantitative analysis of the ratio of the signal intensity of membrane fractions compared with total proteins (P2 + P13 + P100/total, total = P2 + P13 + P100 + S100) in the immunoblot shown in (A). Three independent experiments were performed. Values are mean ± sd.
(C) Quantitative analysis of the ratio of signal intensity of each protein in P13 compared with total protein in the immunoblot shown in (A). **P < 0.01 (n = 3 independent experiments, Student’s t test).
(D) Fluorescence observation of the ERES status (marked by AtSar1b-GFP) in root cells of the wild type and the gpa4-2 mutant. Bars = 10 µm.
(E) In vivo co-IP assay showing that reduced amounts of Sec23c were precipitated in gpa4-1 mutant compared with the wild type. Immunoblots with anti-PDIL1-1 and anti-Sec12b antibodies showing no ER lumenal and membrane protein contamination in the immunoprecipitation samples.
(F) A working model depicting the function of GOT1B in the formation of COPII vesicles. GOT1B participates in COPII coat formation at the ERESs via interaction with Sec23c. The heterodimer of Sec23/Sec24 is recruited by GOT1B and Sar1 cooperatively to form the prebudding complexes in which proglutelin cargos are loaded. Then, the heterotetrimer of Sec13/Sec31 is recruited to form the outer coat of the COPII vesicles before vesicle budding and subsequent fusion with the cis-Golgi apparatus.