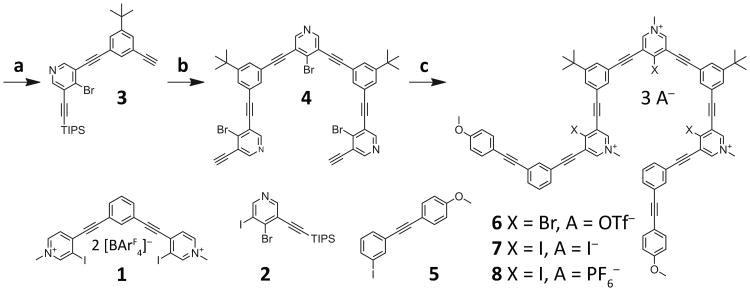
Scheme 1.
Synthesis of the bromo- and iodopyridinium nonamers. Reagents and conditions: a) 2, 1-tert-butyl-3,5-diethynylbenzene, PdCl2(PPh3)2, CuI, Et3N, DMF, RT, 12 h, 21%; b) 4-bromo-3,5-diiodopyridine, PdCl2(PPh3)2, CuI, Et3N, DMF, 50 °C, 12 h, 75%; then TBAF, THF, 0 °C to RT, 10 min, quantitative; c) 5, PdCl2(PPh3)2, CuI, Et3N, DMF, 50 °C, 24 h, 61%; then MeOTf, DCM, RT, 12 h, 93% (6); then NaI, 1:3 v/v DMF-MeCN, RT, 12 h, 90% (7); then AgPF6, 1:1 v/v DMF-EtOAc, 30 min, RT, 80% (8).