Abstract
Background and Aims It is frequently assumed that phenotypic plasticity can be very advantageous for plants, because it may increase environmental tolerance (fitness homeostasis). This should, however, only hold for plastic responses that are adaptive, i.e. increase fitness. Numerous studies have shown shade-induced increases in specific leaf area (SLA), and there is wide consensus that this plastic response optimizes light capture and thus has to be adaptive. However, it has rarely been tested whether this is really the case.
Methods In order to identify whether SLA plasticity does contribute to the maintenance of high biomass of plant species under shaded conditions, a meta-analytical approach was employed. The data set included 280 species and 467 individual studies from 32 publications and two unpublished experiments.
Key Results Plants increased their SLA by 55·4 % on average when shaded, while they decreased their biomass by 59·9 %. Species with a high SLA under high-light control conditions showed a significantly greater ability to maintain biomass production under shade overall. However, in contrast to the expectation of a positive relationship between SLA plasticity and maintenance of plant biomass, the results indicated that species with greater SLA plasticity were less able to maintain biomass under shade.
Conclusions Although a high SLA per se contributes to biomass homeostasis, there was no evidence that plasticity in SLA contributes to this. Therefore, it is argued that some of the plastic changes that are frequently thought to be adaptive might simply reflect passive responses to the environment, or result as by-products of adaptive plastic responses in other traits.
Keywords: Adaptive, functional traits, phenotypic plasticity, leaf mass area, LMA, low light environment, shade tolerance
INTRODUCTION
Phenotypic plasticity is the ability of an organism to express different phenotypes in different environments, and is ubiquitous among organisms (Bradshaw, 1965, 1973; Schmid, 1992; West-Eberhard, 2003). Plants exhibit plasticity in numerous ecologically important traits related to plant function, development and life history (Sultan, 2000; Valladares et al., 2007; Gratani, 2014). It is frequently assumed that phenotypic plasticity can be very advantageous for plants (Baker, 1974; Richards et al., 2006), because it is thought to increase environmental tolerance (i.e. fitness homeostasis; Valladares et al., 2014). This should, however, only hold for plastic responses that are adaptive, i.e. increase fitness (van Kleunen and Fischer, 2005; Muth and Pigliucci, 2007; van Kleunen et al., 2011). Although many studies demonstrated that certain plastic responses of plants to contrasting environments are adaptive (Poorter and Lambers, 1986; Valladares and Pearcy, 1998; Donohue et al., 2001), this is not always the case, as some plastic responses might also be neutral (i.e. do not affect fitness) or even maladaptive (i.e. decrease fitness; van Kleunen and Fischer, 2005; Sánchez-Gómez et al., 2006; Ghalambor et al., 2007). Therefore, it is important to assess explicitly whether the plasticity of a trait is adaptive or not by investigating its contribution to the performance of plants in multiple environments.
Light, one of the crucial factors for the growth and development of plants, is a highly heterogeneous environmental resource in nature, and almost all plants are exposed to a certain degree of shading during their lifetime (Valladares and Niinemets, 2008). At low light intensity, photosynthesis and, consequently, plant growth, are reduced. Plants respond to changing light conditions by adjusting a suite of morphological and physiological traits, such as specific leaf area (SLA), internode and petiole lengths, leaf size, leaf thickness, leaf mass and chlorophyll content (Rozendaal et al., 2006; Valladares and Niinemets, 2008; Legner et al., 2014). While it is frequently implicitly assumed that these morphological and physiological changes are active plastic response to alleviate the effects on the plant of environmental stress, they could also reflect passive plastic responses to reduced resource availability (van Kleunen and Fischer, 2005).
Specific leaf area, the ratio of leaf area to leaf dry mass, is a key functional trait of plants underlying variation in growth rate among species (Pérez-Harguindeguy et al., 2013). SLA is also a major trait in the worldwide leaf economics spectrum, which reflects the range of fast to slow returns on nutrient and dry mass investment in leaves among species (Wright et al., 2004; Flores et al., 2014). Plants usually develop a higher SLA when grown under low-light conditions (Reich et al., 2003; Rozendaal et al., 2006; Feng and van Kleunen, 2014). This response could help plants to increase the efficiency of light capture and maximize carbon gain in such environments (Evans and Poorter, 2001; Gommers et al., 2013), because SLA tends to scale positively with the mass-based light-saturated photosynthetic rate (Pérez-Harguindeguy et al., 2013). Therefore, it is generally assumed that the plastic response of SLA enables plants to maintain a high performance under shading, and has to constitute adaptive plasticity (Valladares and Niinemets, 2008; van Kleunen et al., 2011; Feng and van Kleunen, 2014). However, few studies have tested explicitly whether plastic responses to shading in SLA are really adaptive (but see Steinger et al., 2003; Avramov et al., 2006; Sánchez-Gómez et al., 2006; McIntyre and Strauss, 2014 for notable exceptions), and thus result in a high performance of plants across different light intensities.
Here, we employed a meta-analytical approach to test whether plasticity of SLA in response to shading is adaptive, i.e. whether it enables plants to maintain their fitness under shade conditions. Fitness is ideally measured in terms of reproductive output; however, few studies have quantified this. Biomass is an alternative measure of plant performance, as it is the direct product of growth (e.g. Dawson et al., 2012), and thus the change in biomass between high- and low-light conditions offers a good proxy for a species’ ability to tolerate shade. We compiled a database of 467 studies from 32 publications and two unpublished experiments that measured the responses of biomass and SLA of 280 plant species to shading to test whether greater plastic changes in SLA in response to shading actually help the plants to better maintain performance under shade (i.e. whether plasticity in SLA is positively related to maintenance of plant biomass).
MATERIALS AND METHODS
Study and data collection
As a basis for the meta-analysis, we used a data set from a previous meta-analysis by Dawson et al. (2012), which was on the relationship between resource use and global naturalization success of plants. This data set included 15 studies on this topic published between 1990 and 2009. To obtain more recent studies (i.e. covering 2010–2014) on SLA and performance responses of plants to shading, we conducted a literature search in Web of Science (http://apps.webofknowledge.com/) using the following search string ‘shad*’ OR ‘light*’ OR ‘R:FR’ OR ‘PAR’ AND ‘SLA’ OR ‘LMA’ OR ‘SLM’. In order to ensure that we did not miss any important studies, we also did a similar search in Google Scholar using the same keywords. Our searches resulted in 1055 new records. We then individually assessed each publication, and retained them if the study reported data on both plant biomass and SLA responses to shading. In total, we identified 33 publications that met our criteria (see Supplementary Materials and Methods S1 for all publications used), covering 113 species and 280 individual studies. We also added unpublished data from two of our own experiments (D. Prati, unpubl. data; E. Haeuser, W. Dawson and M. van Kleunen, unpubl. data) to the data set, yielding data on an additional 167 species and 187 individual studies.
We extracted mean values, sample sizes and measures of variance [i.e. s.d., s.e. or 95 % confidence intervals (CIs)] for plant biomass and SLA measures under a high-light control treatment and a shade treatment. We used the high-light treatment as the control treatment because we assumed it to be in the range of light intensities under which photosynthesis is light saturated. We did not consider studies that were performed in growth chambers with artificial lighting, because high-light conditions in growth chambers are much lower than in glasshouse and garden environments, and below the light intensity under which photosynthesis is light saturated. When more than one shading level was used for a single species, they were all included in our analyses (and compared with the same high-light control), but we accounted for multiple measurements per species in the analysis (see below). We extracted the data directly from the text or tables, or, when presented in figures, we extracted the data using the software Image J 1·47v (Rasband, 2013). We also extracted data on light intensity of the high-light control and shade treatments, and calculated the relative light intensity of the shade treatment compared with the control high-light treatment. Because light intensity in glasshouses is typically lower than that outdoors, we also extracted information on whether a study was conducted in a garden experiment or a glasshouse.
Effect size and variance
To examine the effects of shade treatment on SLA and plant biomass, we calculated the log response ratio (lnR) as an effect size of response variables for each individual study following Hedges et al. (1999) as:
Here, and are the mean values of each individual SLA or biomass observation in the shade (S) and control (C) treatments, respectively. LnR values <0 indicate a decrease in SLA or biomass when shaded, and values >0 indicate an increase in SLA or biomass. The variance of lnR was, following Hedges et al. (1999), calculated as
Here, Ns, Nc, s.d.s, s.d.c, and are sample sizes, standard deviations and mean values for SLA or biomass in the shade (S) and control (C) treatments, respectively. As average biomass, and consequently also absolute changes in biomass in response to shading, might vary enormously among species (e.g. an annual herb has a much lower biomass than a tree), we chose the log response ratio as an effect size as it quantifies the proportional change instead of the absolute change in biomass (Hedges et al., 1999).
Data analysis
All meta-analytical calculations and statistical analyses were performed in R 3.1.3 (R Core Team, 2015) using the package Metafor v1.9-5 (Viechtbauer, 2010). To test whether plastic changes in SLA in response to shading actually help the plant to better maintain performance (i.e. biomass) under shade, we selected a multivariate meta-analytical model using the rma.mv function. In the model, we included the effect sizes (lnR) of biomass and their corresponding sampling variances as the response variable. As the main explanatory variable of interest, we included plasticity of SLA in response to shading (i.e. SLAshade – SLAcontrol) in the model. Because the change in biomass may also depend on the SLA under high-light control conditions (SLAcontrol), we also included this baseline SLA as an explanatory variable in the model. Effectively, by including both SLAcontrol and (SLAshade – SLAcontrol), we included both standard parameters (the intercept and slope) of a species linear SLA reaction norm to shading. We chose SLA under high-light conditions as the baseline (intercept) instead of SLAshade, because the high-light conditions were likely to be more similar among studies than the low-light conditions. Moreover, while SLAshade was strongly correlated with (SLAshade – SLAcontrol) (Pearson r = 0·812, P < 0·001, n = 467), resulting in multi-collinearity problems when including both variables in a single analysis, this was not the case for SLAcontrol and (SLAshade – SLAcontrol) (Pearson r = 0·084, P = 0·069, n = 467), despite a strong correlation between SLAshade and SLAcontrol (Pearson r = 0·650, P < 0·001, n = 467). As species varied in life form and studies varied in the degree of shading imposed, and in whether the study was done outdoors or in a glasshouse, we also included life form (woody vs. non-woody), relative light intensity (proportion of light in shade treatment compared with high-light control treatment) and experiment type (garden vs. glasshouse) as explanatory variables. The continuous explanatory variables (SLAshade – SLAcontrol, SLAcontrol and relative light intensity) were all standardized by subtracting the mean and dividing by the s.d. for the entire data set, to facilitate interpretation and comparisons of the estimated model parameters (Schielzeth, 2010).
As effect sizes on the same species and from the same study are not independent, we included species and study as random factors. Moreover, as recent studies have shown that the addition of phylogenetic information could have a significant impact on the effect size estimates from meta-analysis models (Chamberlain et al., 2012), we also included phylogenetic information as a variance–covariance matrix in the model. We first constructed a base phylogenetic tree of all the species in our data set using the online program Phylomatic (Webb and Donoghue, 2005). Polytomies within this base tree were then solved as far as possible using published molecular phylogenies (see Supplementary Materials and Methods S2 for all publications used). The phylogenetic tree was transformed to an ultrametric tree using the compute.brlen function in the package ape v 3.2 (Paradis et al., 2004). Finally, a variance–covariance matrix was calculated from the ultrametric tree, representing phylogenetic relatedness among species, using the vcv function in the package ape v 3.2.
The estimates of effect size of biomass may be affected by whether or not the same genetic plant material is used in both the high-light and shading treatments (Gianoli and Valladares, 2012) and by whether neutral shade (reduced light quantity alone) or canopy shade (reduced light quantity with altered spectral quality) is used (Griffith and Sultan, 2005). However, as in our data set, only six studies used the same genetic material in the different treatments and only three studies used canopy shade in high-light and shade treatments, we did not include these two factors in the main meta-analytical model described above. Instead, we performed separate analyses to test whether material used in each study (replicated genotype or non-replicated genotype) or shade type (neutral shade or canopy shade) had a significant influence on the estimates of the effect sizes of biomass and SLA in response to shading, using the rma.mv function. We included species and study in the model as random factors, and phylogeny as a variance–covariance matrix. We also performed separate analyses to test whether experiment type (garden or greenhouse) or plant life form (woody or non-woody) had a significant influence on estimates of effect size of biomass and SLA in response to shading.
Using the models described above, we calculated a weighted mean effect size for each moderator. We calculated 95 % CIs with 1000 bootstrap replications, using the boot.ci function in the package boot v1.3-15 (Canty and Ripley, 2015). We considered the mean effect size estimate to be significantly different from zero if the 95 % CI around the mean did not include zero. In order to visualize the relationship between the plasticity of SLA and the changes in plant biomass in response to shading, we plotted all biomass effect sizes against SLA plasticity values, and added the regression line based on the predicted values from the main meta-analytical model described above. Total heterogeneity (QT) in the models used for separate analyses can be partitioned into heterogeneity explained by the model structure (QM) and unexplained heterogeneity (QE). We used the QM test to determine the significance of the difference in the mean effect size between different levels in the following moderator variables: plant material type (replicated genotype or non-replicated genotype), shade type (neutral shade or canopy shade), experiment type (garden or greenhouse) and plant life form (woody or non-woodly). Because residual plots revealed a deviation from the assumption of normality, we used randomization tests to obtain a robust significance level of differences between groups (QM). By performing 1000 iterations for each model, a frequency distribution of possible QM values was generated. We then compared the randomly generated values with the observed QM value of each model, and calculated the proportion of randomly generated QM values more extreme (equal to or larger) than the observed QM values. We used this proportion as the significance level (i.e. P-value) for differences between groups.
RESULTS
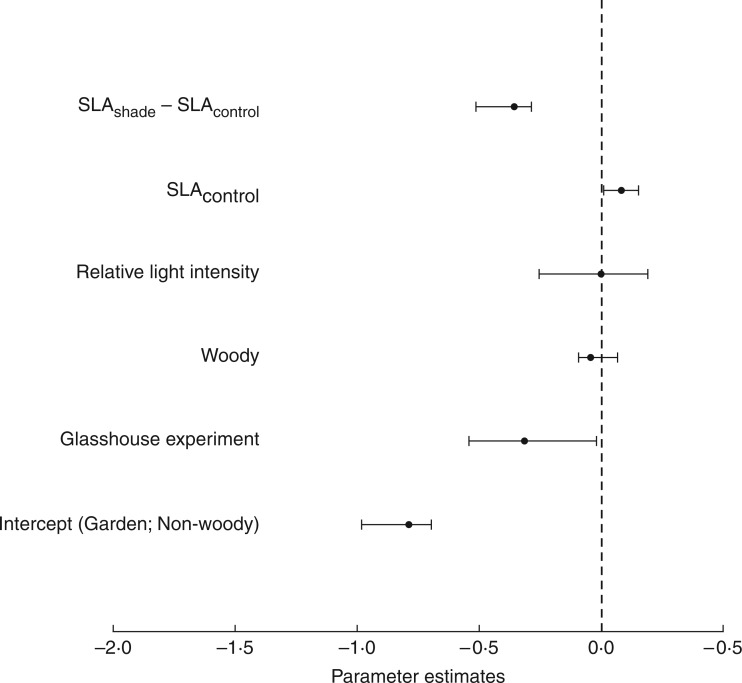
On average, SLA of plants increased by 55·4 % when shaded, while biomass decreased by 59·9 % (Fig. 1). The responses of SLA and biomass to shading were not significantly affected by shade types (neutral or canopy), plant material type (replicated genotype or non-replicated genotype), experiment type (garden or greenhouse) or life form (woody or non-woody) (Fig. 1; Supplementary Data Table S1). The level of light in the shade treatment, relative to the high-light control treatment (mean, 41·5 %; range, 1–85·3 %) had no significant effect on the reduction in biomass (Fig. 2). Species with a greater SLA under control conditions (i.e. high light) showed a significantly smaller decrease in biomass under shade vs. control conditions overall (Figs 2 and 3). However, we found a negative relationship between SLAshade – SLAcontrol and lnR of biomass (Figs 2 and 3). In other words, the decrease in biomass under shading was significantly greater for plant species that showed a greater plastic increase in SLA. The variance component associated with phylogenetic history was low (0·0446), indicating that the effect sizes used in the analysis were not strongly phylogenetically related.
Fig. 1.
Mean effect sizes (log response ratio) describing the overall responses of biomass and SLA to shading, and how these responses depend on whether the species are woody or non-woody, and whether the study was done in a glasshouse or garden, used the same genetic material in the different light treatments, and used neutral or canopy shading. Error bars represent bias-corrected bootstrapped 95 % confidence intervals around the mean effect size estimates derived from the phylogenetically corrected meta-analytical model. The sample sizes (i.e. the number of studies) are given in parentheses. The dashed line indicates zero effect of shading.
Fig. 2.
Means of parameter estimates describing the relationship between biomass responses to shading [ln(biomassshade/biomasscontrol)] and SLA plasticity in response to shading (i.e. SLAshade – SLAcontrol), SLA in the high-light control treatment (SLAcontrol), relative light intensity (percentage light in shade treatment relative to high-light control treatment) and type of experiment (garden vs. glasshouse) on the changes of plant biomass in response to shading. Error bars show the bias-corrected bootstrapped 95 % confidence intervals around the parameter estimates derived from the phylogenetically corrected meta-analytical model. The dashed line indicates zero effect of the respective explanatory variable.
Fig. 3.
Relationship between changes in plant biomass in response to shading, and (A) SLA in the high-light control treatment (SLAcontrol, i.e. the intercept of the species’ reaction norm) and (B) the changes in SLA (i.e. the slope of the species’ reaction norm). The regression line is based on the predicted values from the phylogenetically corrected meta-analytical model. The solid line is the fitted line, and the dashed lines are 95 % confidence intervals of the fitted line.
DISCUSSION
Specific leaf area is considered to be an important functional trait that may affect light interception and leaf longevity (Wright et al., 2004), and is highly plastic in response to shading (Valladares and Niinemets, 2008). Although it is known that not all phenotypic plasticity increases performance (van Kleunen and Fischer, 2005), it is still frequently implied that plasticity in SLA should help plants maintain high performance under varying light conditions (van Kleunen et al., 2011; Gratani, 2014). Surprisingly, however, we found that greater plasticity of SLA of a species in response to shading was not associated with the maintenance of plant performance, but rather with greater reductions in plant biomass. Therefore, the results of our meta-analysis indicate that SLA plasticity to shading might not constitute adaptive plasticity.
Confirming the results of numerous previous studies on plant responses to shading (Reich et al., 2003; Rozendaal et al., 2006; Gianoli and Saldana, 2013; Feng and van Kleunen, 2014), our meta-analysis showed that most plants produced leaves with a higher SLA when shaded. This plastic response of SLA results in thinner, and relatively larger, leaves, and consequently should enhance light capture per gram of leaf tissue and thus mass-based photosynthesis. Therefore, it is frequently assumed that SLA plasticity represents adaptive shade tolerance plasticity, maximizing plant performance in the shade (Valladares and Niinemets, 2008; van Kleunen et al., 2011; Freschet et al., 2015). However, in contrast to support for this general assumption, we found a negative relationship between plant biomass responses to shading and SLA plasticity. In other words, our findings indicate that species that increased their SLA to a larger degree in response to shading were not more but less shade tolerant, compared with species that hardly changed their SLA.
Few other studies have tested explicitly whether shade-induced responses in SLA are adaptive. Avramov et al. (2006) tested the adaptive value of plasticity in SLA of plants from two populations of Iris pumila grown at three light levels, and found evidence that the plastic response in SLA to light availability was in the direction of values favoured by selection in one of the two populations (i.e. adaptive). Moreover, McIntyre and Strauss, (2014) investigated patterns of plasticity and selection on SLA of Claytonia perfoliata plants grown in an oak canopy understorey and an adjacent grassland habitat, and found that C. perfoliata exhibited plastic responses in SLA in the same direction as promoted by selection (i.e. selection for a higher SLA in a canopy habitat), suggesting that the plastic reponse in SLA is adaptive. These two results thus contrast with the findings of our meta-analysis. One possible explanation for the discrepancy might be that these other studies tested for the benefit of plasticity within species, while we tested for the benefit of plasticity among species. Therefore, we clearly need more studies that assess the fitness effects of SLA plasticity in response to shading within species to see whether this plasticity is generally beneficial within species.
Our findings do not just suggest that a strong plastic increase in SLA of a species in response to shading is non-adaptive, but even suggest that it is maladaptive. One possible explanation could be that SLA plasticity is genetically and developmentally linked to plasticity in shade-avoidance traits, such as petiole and internode elongation. In contrast to a shade-tolerance trait, a shade-avoidance trait should help the plants to escape from the shade conditions by overtopping the neighbouring plants that impose the shade or by finding gaps in the vegetation. However, as most experiments on shade responses use artificial shading treatments from which the plants cannot escape, elongation responses are futile and might even be costly (Valladares et al., 2007; Valladares and Niinemets, 2008). Another explanation for the negative association between SLA plasticity and biomass homeostasis could be that most studies measure SLA at the end of the experiment. If SLA determines light interception per gram of leaf, then plants that are able plastically to adjust SLA early should be able to maintain a high biomass production. However, SLA as measured at the end of an experiment might not be driving the performance of plants but might result from it. In other words, a plant that is not very shade tolerant, and thus shows a strong decrease in biomass in response to shading, will not have the resources (e.g. photo-assimilates) to produce thick leaves with a low SLA. A low SLA might be beneficial, also under shaded conditions, if it results in a greater proportion of incident photon capture per unit leaf area. Alternatively, it could be that plants do not actively increase their SLA in response to low light but instead passively decrease their SLA in response to high light due to accumulation of non-structural carbohydrates (thus increasing dry mass per leaf area) when the carbohydrate production exceeds the demand in meristems. Whatever the exact reason is for the negative association between SLA plasticity and biomass homeostasis, we recommend that future studies on this topic should measure SLA not only at the end of an experiment but also early on, and that they should impose more realistic shade treatments that allow shade-avoidance responses to be effective.
While our results indicate that SLA plasticity in response to shading is not adaptive, one could argue that our results indicate that SLA plasticity is adaptive in response to an increase in light intensity. In other words, if one uses the shade environment as the reference instead of the high-light environment, the plant species that have a stronger plastic decrease in SLA in response to high light are better able to take advantage of the high light intensity in terms of biomass production (Supplementary Data Fig. S1a). To gain more insight into the underlying cause of the relationship between biomass change and SLA plasticity, we also carried out a regression of biomass in high- and low-light environments separately against SLA plasticity (Supplementary Data Fig. S1b). Plant biomass in high-light environments varied little in relation to SLA plasticity (Fig. S1b), but biomass under low-light environments decreased with increasing SLA plasticity (Fig. S1b). This indicates that species with greater SLA plasticity do not have an advantage under high-light conditions, but are disadvantaged under shade compared with less plastic species. In other words, the reduced ability of plants to produce biomass due to a lack of light in shaded environments is not compensated by increasing SLA to a greater degree, but is rather exacerbated by it.
Although SLA plasticity did not help plants to maintain a high performance when shaded, our results showed that species with greater SLA under high-light control conditions have a significantly smaller decrease in biomass when shaded. So, while plasticity in SLA did not increase biomass homeostasis, high SLA values did. Generally, shade-intolerant species have higher light compensation points and light-saturated photosynthetic rates (Givnish, 1988; Kitajima, 1994; Valladares and Niinemets, 2008), thus plants with high SLA values would be more shade tolerant. This finding supports the carbon-gain hypothesis, which states that any trait related to light-use efficiency that improves carbon gain in plants will increase performance under shade (Givnish, 1988; Valladares and Niinemets, 2008). Our finding is also in line with the many studies that found that species with a greater SLA are more shade tolerant (e.g. Sánchez-Gómez et al., 2006; Janse-Ten Klooster et al., 2007; Gianoli and Saldana, 2013). Although the relationship between the biomass response and SLAcontrol in our meta-analysis was shallow, it raises the question of why not all species have evolved greater SLA. Most probaby, this is because some species do not encounter much shading in nature, and other selective forces, such as herbivory and drought stress, and environments favouring leaf longevity (Supplementary Data Fig. S2), have resulted in the evolution of species with low SLA. Additionally, while plants with lower SLA are less efficient in terms of metabolic cost per unit leaf area, they might capture a greater proportion of incident photons. When the increased photon capture more than offsets the increased metabolic cost of a lower SLA, the lower SLA should be favoured.
As species that naturally occur in shaded habitats are presumably more shade tolerant, it could be that the positive relationship between the change in biomass and SLA arose because species from shade habitats have higher SLA values than species from non-shade habitats. As information on the natural habitats is not available for most of the study species, we could not account for this in the main analysis. However, for 136 of the 280 study species, we had data on their Ellenberg light-indicator values (Ellenberg, 1974), which indicate the light conditions in the natural habitat of the species in Europe. Although this sub-set of species did not contain species from deep-shade habitats, we did not find evidence that species with different light-indicator values differed in SLA under high-light and under shaded conditions (Supplementary Data Fig. S3). Therefore, it is unlikely that our result of a higher biomass homeostasis for species with higher SLA values is confounded by species from shade habitats having higher SLA values.
Surprisingly, our results showed that relative light intensity had no significant effect on the reduction in biomass (Fig. 2). This runs counter to the results of many experiments, where biomass typically declines more or less continuously with declining light levels (e.g. Feng and van Kleunen, 2014; Kumar et al., 2014; Konvalinková et al., 2015). A likely explanation for this apparent discrepancy is that most species in our meta-analysis were not grown under more than two experimental light conditions, and that the light conditions varied among studies. Seventy of the 280 species were grown under more than two light levels, and a post-hoc analysis for this subset of species showed that within species, biomass declines more or less continuously with declining light levels (Supplementary Data Fig. S4). However, if we run the full meta-analytical model for this sub-set of 70 species, the effect of relative light intensity was still not significant, and the other results also remained qualitatively the same (Supplementary Data Fig. S5). So, while within each species relative light intensity is important for the change in biomass, among species it plays no significant role.
Conclusions
In summary, our meta-analysis suggests that plasticity in the ability of plants to capture more light per gram of leaf mass invested under low-light conditions by increasing SLA does not contribute to shade tolerance of plant species in terms of biomass homeostasis, and thus does not constitute adaptive phenotypic plasticity. This is despite wide consensus that plasticity in SLA and other traits associated with shade avoidance and tolerance, such as leaf length, leaf area, shoot–root ratio, chlorophyll content and photosynthesis, can be adaptive (Dudley and Schmitt, 1996; Schmitt et al., 1999; van Kleunen and Fischer, 2005; Valladares and Niinemets, 2008; van Kleunen et al., 2011). We argue that some of the plastic responses of plant species to shade that are frequently thought to be adaptive might simply reflect passive responses to the environment, or represent by-products of adaptive plastic responses in other traits. In order to understand further the mechanism of plant shade tolerance, we therefore strongly recommend that future studies should explicitly test whether the plasticity of a trait is adaptive or not.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Methods S1: list of published studies from which data on plastic changes in biomass and SLA in response to shading were extracted. Methods S2: phylogenetic tree used in this study and list of published studies used for resolving the polytomies within the initial base tree. Table S1: mean effect size estimates from phylogenetically corrected meta-analytical models performed separately for each factor. Figure S1: relationship between plasticity in SLA and the changes in plant biomass when going from shade to high-light control environments, and plant biomass under different light conditions. Figure S2: means (± s.e.) of SLA in the ligh-light control treatments as estimated from a liner mixed-effects model for evergreen woody, deciduous woody and non-woody (herbaceous) species. Figure S3: boxplots of SLA values and biomass response to shading of species of different shade intolerance classes. Figure S4: the relationship between plant biomass reduction and relative light intensity for 70 species that were grown under more than two light conditions. Figure S5: means of parameter estimates describing the relationship between biomass responses to shading and SLA plasticity in response to shading, SLA in the high-light control treatment, relative light intensity and type of experiment on the changes of plant biomass in response to shading.
ACKNOWLEDGEMENTS
We are very grateful to Dr Judy Simon who kindly provided data. We also thank to Dr Liam R. Dougherty and Dr Dylan Craven for their help in carrying out multivariate meta-analysis in R. Y.J.L. is funded by a scholarship from the China Scholarship Council. We thank the editors and two anonymous referees for the valuable comments and suggestions on a previous version of the manuscript.
LITERATURE CITED
- Avramov S, Pemac D, Tucić B. 2006. Phenotypic plasticity in response to an irradiance gradient in Iris pumila: adaptive value and evolutionary constraints. Plant Ecology 190: 275–290. [Google Scholar]
- Baker HG. 1974. The evolution of weeds. Annual Review of Ecology and Systematics 5: 1–24. [Google Scholar]
- Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155. [Google Scholar]
- Bradshaw AD. 1973. Environment and phenotypic plasticity. Brookhaven Symposia in Biology 25: 75–94. [Google Scholar]
- Canty A, Ripley B. 2015. boot: Bootstrap R (S-Plus) Functions. R package version 1.3-15.
- Chamberlain SA, Hovick SM, Dibble CJ, et al. 2012. Does phylogeny matter? Assessing the impact of phylogenetic information in ecological meta-analysis. Ecology Letters 15: 627–636. [DOI] [PubMed] [Google Scholar]
- Dawson W, Rohr RP, van Kleunen M, Fischer M. 2012. Alien plant species with a wider global distribution are better able to capitalize on increased resource availability. New Phytologist 194: 859–867. [DOI] [PubMed] [Google Scholar]
- Donohue K, Pyle EH, Messiqua D, Heschel MS, Schmitt J. 2001. Adaptive divergence in plasticity in natural populations of Impatiens capensis and its consequences for performance in novel habitats. Evolution 55: 692–702. [DOI] [PubMed] [Google Scholar]
- Dudley SA, Schmitt J. 1996. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. American Naturalist 147: 445–465. [Google Scholar]
- Ellenberg H. 1974. Zeigerwerte der Gefäßpflanzen Mitteleuropas. Scripta Geobotanica 9: 1–97. [Google Scholar]
- Evans JR, Poorter H. 2001. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell & Environment 24: 755–767. [Google Scholar]
- Feng Y, van Kleunen M. 2014. Responses to shading of naturalized and non-naturalized exotic woody species. Annals of Botany 114: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores O, Garnier E, Wright IJ, et al. 2014. An evolutionary perspective on leaf economics: phylogenetics of leaf mass per area in vascular plants. Ecology and Evolution 4: 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Swart EM, Cornelissen JH. 2015. Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytologist 206: 1247–1260. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- Gianoli E, Saldana A. 2013. Phenotypic selection on leaf functional traits of two congeneric species in a temperate rainforest is consistent with their shade tolerance. Oecologia 173: 13–21. [DOI] [PubMed] [Google Scholar]
- Gianoli E, Valladares F. 2012. Studying phenotypic plasticity: the advantages of a broad approach. Biological Journal of the Linnean Society 105: 1–7. [Google Scholar]
- Givnish TJ. 1988. Adaptation to sun and shade – a whole-plant perspective. Australian Journal of Plant Physiology 15: 63–92. [Google Scholar]
- Gommers CMM, Visser EJW, Onge KRS, Voesenek LACJ, Pierik R. 2013. Shade tolerance: when growing tall is not an option. Trends in Plant Science 18: 65–71. [DOI] [PubMed] [Google Scholar]
- Gratani L. 2014. Plant phenotypic plasticity in response to environmental factors. Advances in Botany 2014: 1–17. [Google Scholar]
- Griffith TM, Sultan SE. 2005. Shade tolerance plasticity in response to neutral vs green shade cues in Polygonum species of contrasting ecological breadth. New Phytologist 166: 141–147. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80: 1150–1156. [Google Scholar]
- Janse-Ten Klooster SH, Thomas EJP, Sterck FJ. 2007. Explaining interspecific differences in sapling growth and shade tolerance in temperate forests. Journal of Ecology 95: 1250–1260. [Google Scholar]
- Kitajima K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419–428. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Fischer M. 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytologist 166: 49–60. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Schlaepfer DR, Glaettli M, Fischer M. 2011. Preadapted for invasiveness: do species traits or their plastic response to shading differ between invasive and non-invasive plant species in their native range? Journal of Biogeography 38: 1294–1304. [Google Scholar]
- Konvalinková T, Püschel D, Janoušková M, Gryndler M, Jansa J. 2015. Duration and intensity of shade differentially affects mycorrhizal growth- and phosphorus uptake responses of Medicago truncatula. Frontiers in Plant Science 6: 65. doi: 10.3389/fpls.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Sharma S, Ramesh K, Pathania V, Prasad R. 2014. Irradiance stress and plant spacing effect on growth, biomass and quality of wild marigold (Tagetes minuta L.) – an industrial crop in western Himalaya. Journal of Essential Oil Research 26: 348–358. [Google Scholar]
- Legner N, Fleck S, Leuschner C. 2014. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees-Structure and Function 28: 263–280. [Google Scholar]
- McIntyre PJ, Strauss SY. 2014. Phenotypic and transgenerational plasticity promote local adaptation to sun and shade environments. Evolutionary Ecology 28: 229–246. [Google Scholar]
- Muth NZ, Pigliucci M. 2007. Implementation of a novel framework for assessing species plasticity in biological invasions: responses of Centaurea and Crepis to phosphorus and water availability. Journal of Ecology 95: 1001–1013. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics, 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167. [Google Scholar]
- Poorter H, Lambers H. 1986. Growth and competitive ability of a highly plastic and a marginally plastic genotype of Plantago major in a fluctuating environment. Physiologia Plantarum 67: 217–222. [Google Scholar]
- Rasband WS. 2013. ImageJ US National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/.
- R Core Team. 2015. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters 9: 981–93. [DOI] [PubMed] [Google Scholar]
- Rozendaal DMA, Hurtado VH, Poorter L. 2006. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Functional Ecology 20: 207–216. [Google Scholar]
- Sánchez-Gómez D, Valladares F, Zavala MA. 2006. Functional traits and plasticity in response to light in seedlings of four Iberian forest tree species. Tree Physiology 26: 1425–1433. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution 1: 103–113. [Google Scholar]
- Schmid B. 1992. Phenotypic variation in plants. Evolutionary Trends in Plants 6: 45–60. [Google Scholar]
- Schmitt J, Dudley SA, Pigliucci M. 1999. Manipulative approaches to testing adaptive plasticity: phytochrome-mediated shade-avoidance responses in plants. American Naturalist 154: S43–S54. [DOI] [PubMed] [Google Scholar]
- Steinger T, Roy BA, Stanton ML. 2003. Evolution in stressful environments II: adaptive value and costs of plasticity in response to low light in Sinapis arvensis. Journal of Evolutionary Biology 16: 313–323. [DOI] [PubMed] [Google Scholar]
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. [DOI] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39: 237–257. [Google Scholar]
- Valladares F, Pearcy RW. 1998. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia 114: 1–10. [DOI] [PubMed] [Google Scholar]
- Valladares F, Gianoli E, Gomez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytologist 176: 749–763. [DOI] [PubMed] [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters 17: 1351–1364. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 36: 1–48 [Google Scholar]
- Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Notes 5: 181–183. [Google Scholar]
- West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York: Oxford University Press. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.