Abstract
Diffuse pulmonary ossification (DPO) is a rare disease with unknown pathogenesis, clinical manifestations, and treatment options. This report describes the diagnosis of DPO in an otherwise healthy 26‐year‐old man with recurrent spontaneous pneumothorax. His father was diagnosed with a similar lung condition in his 30's with computed tomography (CT) images that were strikingly similar to those of the patient. This report suggests that DPO can induce spontaneous pneumothorax and its pathogenesis may have a possible genetic predisposition that needs further research.
Keywords: Genetics, pneumothorax, pulmonary ossification, rare lung disease
Introduction
Diffuse pulmonary ossification (DPO) is a rare disease with only around 160 reported cases in the literature and is usually diagnosed at post‐mortem 1. DPO is characterized by the formation of bone tissue in the lung, primarily affecting the pulmonary interstitium and alveolar spaces, although the pathogenesis remains poorly understood 1, 2. Patients with DPO are mostly asymptomatic, leading to underdiagnosis 1. Only two other reported cases have suggested that DPO may have a hereditary pattern to its pathogenesis 3. We add to this novel field of DPO pathogenesis by describing a case of recurrent pneumothoraces with evidence to suggest a genetic predisposition.
Case Report
A 26‐year‐old Mexican man presented with a sudden onset of dyspnoea and pleuritic chest pain. He denied any recent respiratory infections or chest wall trauma. He was otherwise active and well.
His medical history included two prior spontaneous pneumothoraces – initially on the right side in 2010 that required chest tube drainage, and a subsequent left‐sided pneumothorax in 2014 that required only supplemental oxygen therapy. Prior computed tomography (CT) scans indicated that he had a pulmonary parenchymal abnormality with yet unconfirmed aetiology.
His father was diagnosed with “pulmonary fibrosis” at age 30 on a routine chest X‐ray examination, followed by CT scans. He is now in his 60's and in good health. His father remained asymptomatic, and therefore declined lung biopsies to further characterize his lung condition.
The presenting patient did not have any history of smoking or drug use. He had no history of occupational or environmental exposures.
On physical examination, his vitals were: 123/71 mmHg, 88 beats/min, 16 respirations/min, oxyhaemoglobin saturation of 97% on room air, and temperature of 36.6°C. Respiratory exam was normal with good air entry and no adventitious sounds. Cardiovascular exam was unremarkable.
Laboratory results revealed the following: normal complete blood count, negative autoantibodies and rheumatological work up, normal serum calcium, and normal liver enzymes and liver function tests. Cardiac work up was also negative. Chest X‐ray on admission showed subtle bibasal reticular opacities but no convincing pneumothorax. A subsequent thoracic CT scan following the chest X‐ray showed a small right‐sided pneumothorax, with background subpleural and peribronchovascular opacities predominantly in the posterior dependent distribution areas. Dense partly calcified linear reticulations in these regions were most consistent with dendriform pulmonary ossification (DPO) (Fig. 1). The differential diagnosis would include underlying disease states that may cause pulmonary calcinosis, such as chronic renal failure, hyperparathyroidism, sarcoidosis, amyloidosis, and pulmonary alveolar microlithiasis, which is a rare disease of widespread intra‐alveolar calcium phosphate deposits.
Figure 1.
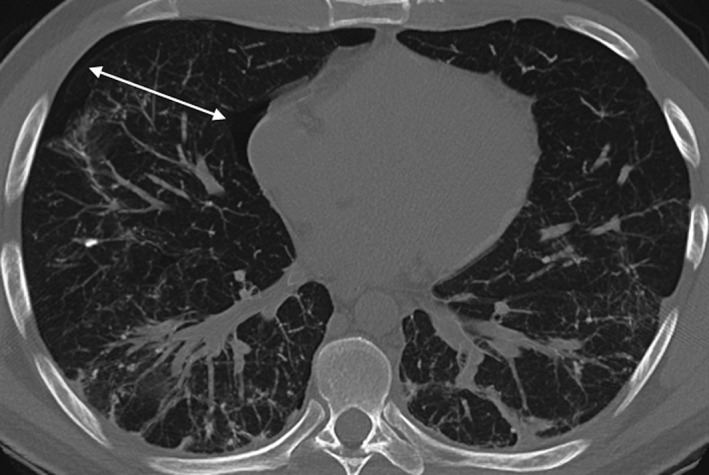
Thoracic computed tomography scan showing a small right‐sided pneumothorax (white arrows), with background subpleural and peribronchovascular opacities predominantly in the posterior dependent distribution areas. Dense partly calcified linear reticulations in these regions were most consistent with dendriform pulmonary ossification.
Previous pulmonary function tests showed normal results with a post‐bronchodilator forced expiratory volume in one second (FEV1) of 91% of predicted.
Bronchoscopy was negative for mycobacterium, granulomatous inflammation, or malignancy.
Because of the repeated episodes of pneumothorax, a thoracoscopic wedge resection of the right upper lobe was performed. The biopsy confirmed DPO, with residual organizing pneumonia. There was no evidence of eosinophilia or granulomatous inflammation, and no classical findings of hypersensitivity pneumonitis (Fig. 2).
Figure 2.

Histopathological section from the surgical lung biopsy showing bony trabeculae (arrow). The dark colouration reflects calcification. The osseous formation appears to arise in association with some fibrous tissue, interpreted as unresolved organizing pneumonia (asterisk). Haematoxylin and eosin, undecalcified section. Original magnification 40×.
The patient was admitted for observation of his pneumothorax, which remained stable during his admission and did not require treatment.
Given the good prognosis of DPO and the lack of any known treatment, the patient will be followed up on an annual basis with no additional treatment.
During one of the follow‐up appointments, the patient's CT scans were compared with his father's, which demonstrated striking similarities. His father's CT scans showed bibasilar thickening of the pulmonary interstitium, reticulation with linear calcifications and subpleural blebs, similar to the presenting patient's CT findings (Fig. 3).
Figure 3.
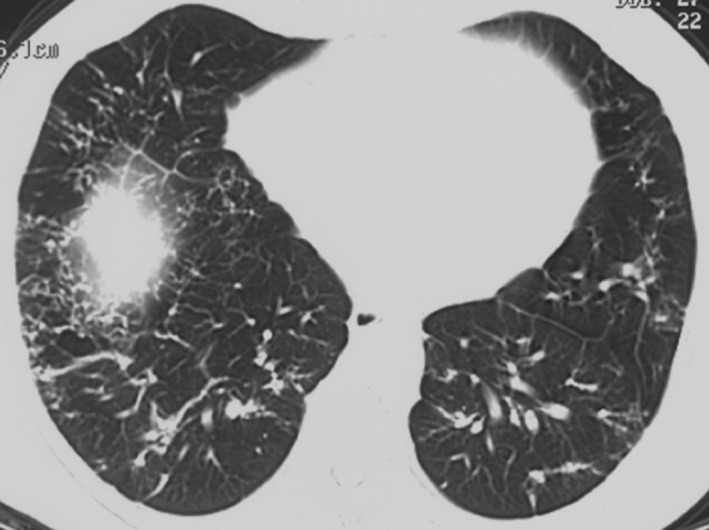
Computed tomography chest scan of the patient's father showing bibasal thickening of the pulmonary interstitium, reticulation with linear calcifications, and subpleural blebs.
Discussion
DPO is a rare disease with an estimated incidence rate of 0.16–0.6% 1, 4. The pathogenesis is still unclear, but has been hypothesized to be secondary to inflammation in the lung, which creates a hypoxic and acidic environment that propagates free radical formation and fibroblastic proliferation, leading to metaplastic ossification 2, 4.
DPO can be divided into two types: nodular and dendriform 1, 2. The nodular form is associated with conditions causing venous congestion, including cardiac diseases such as mitral stenosis 2. The nodular form involves bone fragments within alveolar spaces, and often does not contain marrow 2. The dendriform type is most often associated with pulmonary diseases, including pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and asbestosis 2. It is characterized by serpentine spread of bone, including marrow elements, along the alveolar interstitium and septae 2.
Aside from these predisposing conditions, idiopathic DPO has been described in a few case reports 3, 4. Idiopathic DPO has a predilection for men, especially in the age range of 50–60 2. It usually follows an indolent and slowly progressive course with a good prognosis 2.
There have only been a handful of other reported cases of DPO presenting with spontaneous pneumothorax 3, 5. In one of the cases, a sharp bony spicule near the lung was noted, and theorized to cause the pneumothorax by puncturing the visceral pleura 5.
DPO is a histological diagnosis, and clinical signs and symptoms, laboratory investigations, or radiological findings are often insufficient for diagnosis 2. Radiological findings of DPO (linear calcific densities, sometimes associated with bronchial wall thickening) may be misdiagnosed as bronchiectasis or pulmonary fibrosis 2. There have been recent reports suggesting the use of CT in evaluating smaller foci of lung ossification in order to diagnose DPO in patients who decline open lung biopsies 2.
Our case illustrates a rare entity of DPO – the clinical presentation of recurrent spontaneous pneumothoraces with a possible genetic disposition. The aetiology of DPO in this patient is uncertain; however, the adjacencies of areas of DPO with patches of organizing pneumonia raise the possibility that the DPO lesions may have arisen from certain causes of organizing pneumonia such as recurrent aspiration pneumonia, community acquired pneumonia, or pneumoconiosis or other environmental exposures. Given this patient's otherwise negative past medical history and a lack of significant environmental exposures, and the similarities between his father's CT scans with his own, a genetic predisposition may be considered. The aetiology of our patient's recurrent pneumothoraces may have been secondary to DPO, in which bony spicules could have punctured the visceral pleura. We have included an image from another case of DPO, which showed speculated nodules elevating the pleura (Fig. 4). Other causes of recurrent pneumothoraces, such as cystic or bullous lung disease, trauma, or bronchopleural fistula were not apparent in our patients’ investigations. This provides insight on possible complications of DPO despite its entity as a mostly indolent disease.
Figure 4.

Histopathological section from another case of dendriform pulmonary ossification showing thin bony trabeculae inserting themselves within the alveolar interstitium (arrowheads). The speculated nodule elevates the pleura (arrow), providing some visual indication by which pneumothoraces may occur in these patients. Haematoxylin and eosin, undecalcified section. Original magnification 40×.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
The authors would like to acknowledge Giselle Peschard for her support and insight into the case report, and Sian Hsiang‐Te Tsuei for valuable edits.
Tsai, A.P.Y. , English, J.C. , Murphy, D. and Sin, D. (2017) Recurrent pneumothorax related to diffuse dendriform pulmonary ossification in genetically predisposed individual. Respirology Case Reports, 5 (2), e00211. doi: 10.1002/rcr2.211.
Associate Editor: Arata Azuma
References
- 1. Lara J, Catroppo J, Kim DU, et al. 2005. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification. Report of a 26‐year autopsy experience. Arch. Pathol. Lab. Med. 129:348–353. [DOI] [PubMed] [Google Scholar]
- 2. O'Donnell R, Nicholson S, Meaney J, et al. 2008. A case of persistent pulmonary consolidation. Respiration 75:355–358. [DOI] [PubMed] [Google Scholar]
- 3. Azuma A, and Miyamoto H 2003. Familial clustering of dendriform pulmonary ossification. Sarcoidosis Vasc. Diffuse Lung Dis. 20:152–154. [PubMed] [Google Scholar]
- 4. Tseung J, and Duflou J 2006. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology (Phila.) 38(1):45–48. [DOI] [PubMed] [Google Scholar]
- 5. Kato T, Ishikawa K, Kadoya M, et al. 2012. Spontaneous pneumothorax in a patient with dendriform pulmonary ossification: report of a case. Surg. Today 42:903–908. [DOI] [PubMed] [Google Scholar]


