FIG 8 .
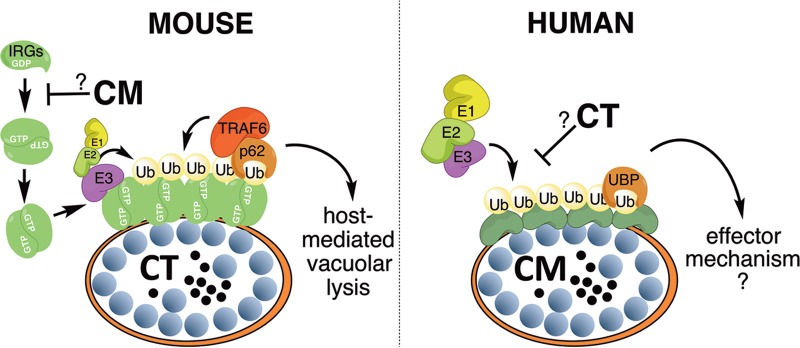
Distinct pathways in mice and humans control IFN-γ-inducible ubiquitination of inclusions. Inclusion ubiquitination in mouse cells is dependent on IFN-γ-inducible IRGs, which in their GTP-bound state form oligomers, bind to inclusions, and subsequently recruit E3 ubiquitin ligases such as TRAF6. By interfering with the recruitment of IRGs to its inclusion, the rodent pathogen C. muridarum (CM) blocks inclusion ubiquitination in mouse cells. Ubiquitinated inclusions undergo vacuolar lysis, leading to bacterial death (25). This current study demonstrates that IFN-γ priming also triggers inclusion ubiquitination in human cells, albeit by an IRG-independent mechanism. Whereas C. muridarum is susceptible to this IRG-independent pathway, the human pathogen C. trachomatis (CT) is resistant. Inclusion ubiquitination in human cells correlates with the elimination of inclusions from infected cells, but the underlying cellular mechanism is unknown. Ub, ubiquitin; UBP, ubiquitin-binding proteins; E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme.