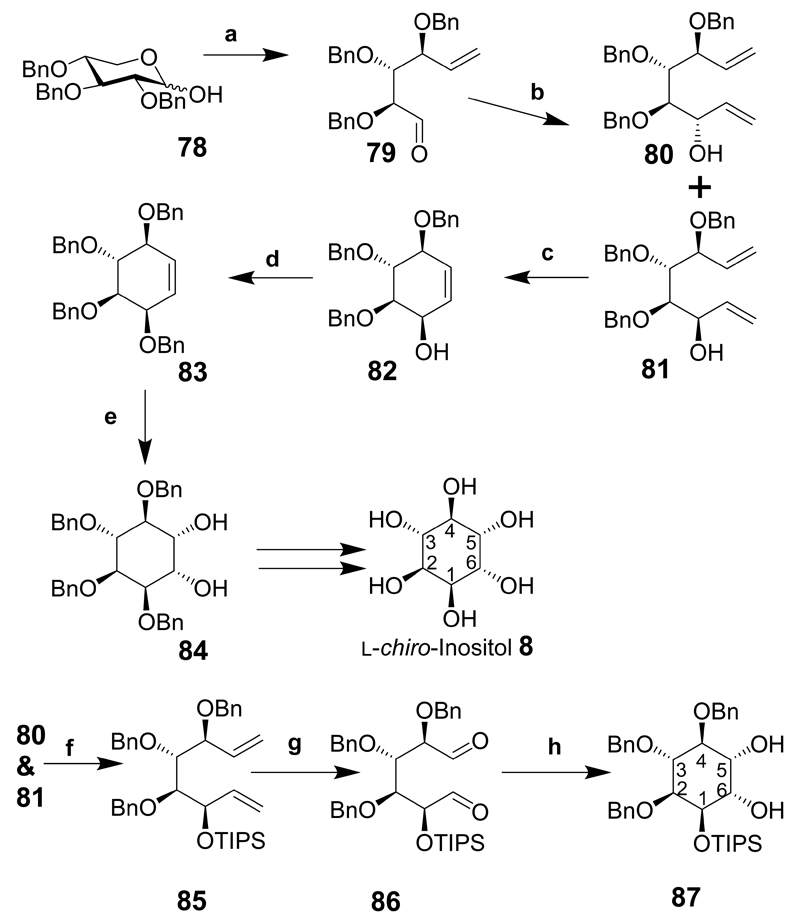
Scheme 11.
Reaction conditions: (a) CH2=PPh3, THF, 45°C, 10 h; then COCl2, Me2SO, CH2Cl2, –78°C, 20 min, Et3N, –78°C to rt; (b) vinyl magnesium bromide, MgBr2·OEt2, –78°C, CH2Cl2, 3 h; (c) (CyP)2RuCl2(CHPh), 10 mol %, CH2Cl2, 15 min, 99%; (d) BnBr, DMF, NaH, 94%; (e) OsO4, NMO, Me2C=O/H2O, 93%; (f) TIPS-Cl, DMF, AgNO3, Separate compounds, (yield not given for this step, but 54% over 3 preceding steps); (g) O3, CH2Cl2, pyridine, then Me2S, (h) SmI2, tert-BuOH, THF, –78°C, 3 h, then 20°C, O/N.