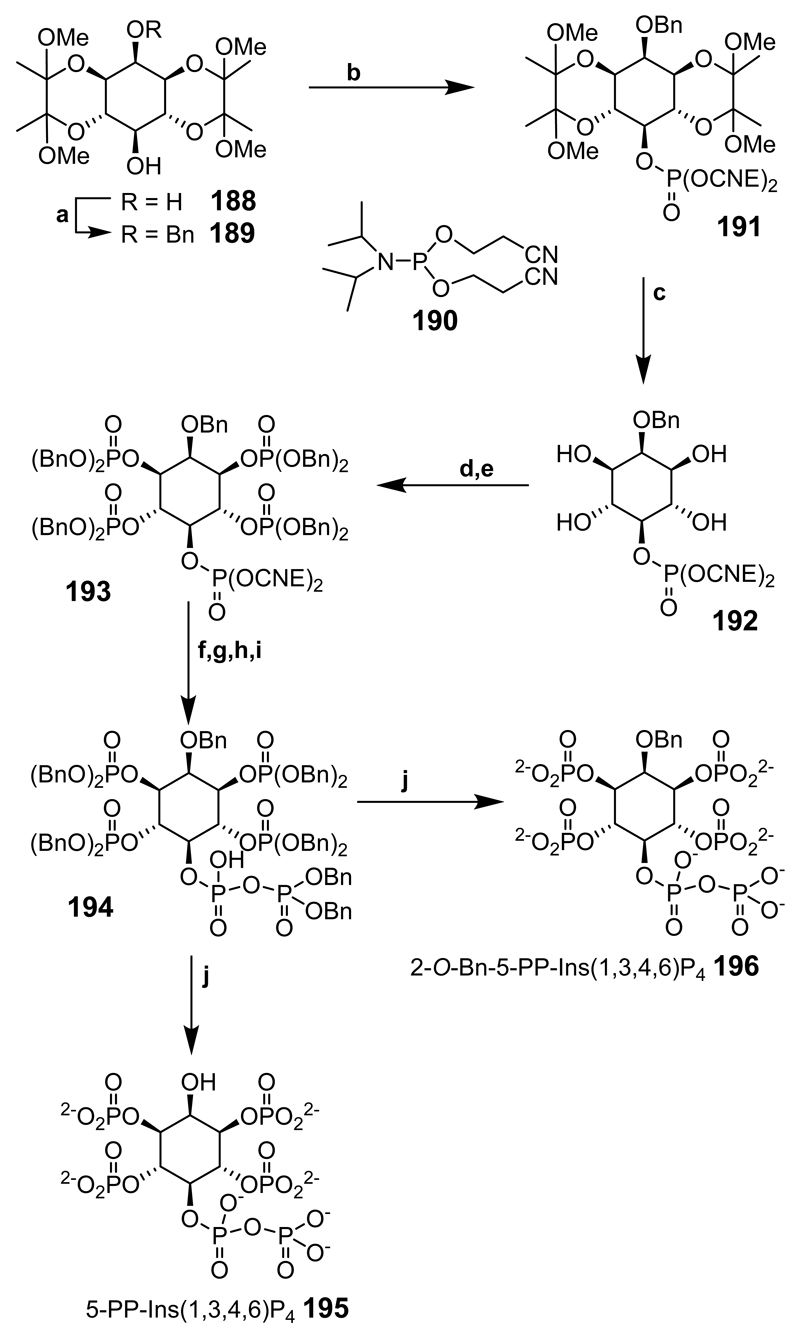
Scheme 25.
Reaction conditions: (a) BnBr, NaH, DMF; (b) reagent 190, CH2Cl2, 5-Ph-1H-tetrazole, then MCPBA, –40°C→rt; (c) 90% TFA(aq), 1:1; (d) reagent 173, CH2Cl2, 5-Ph-1H-tetrazole; (e) MCPBA, –40°C→rt; (f) DBU then BSTFA; (g) TFA, MeOH; (h) reagent 173, CH2Cl2, 5-Ph-1H-tetrazole; (i) MCPBA, –40°C→rt; (j) Pd(OH)2, H2, tert-BuOH, H2O (196 was prepared in the presence of DBU to inhibit O-benzyl deprotection).