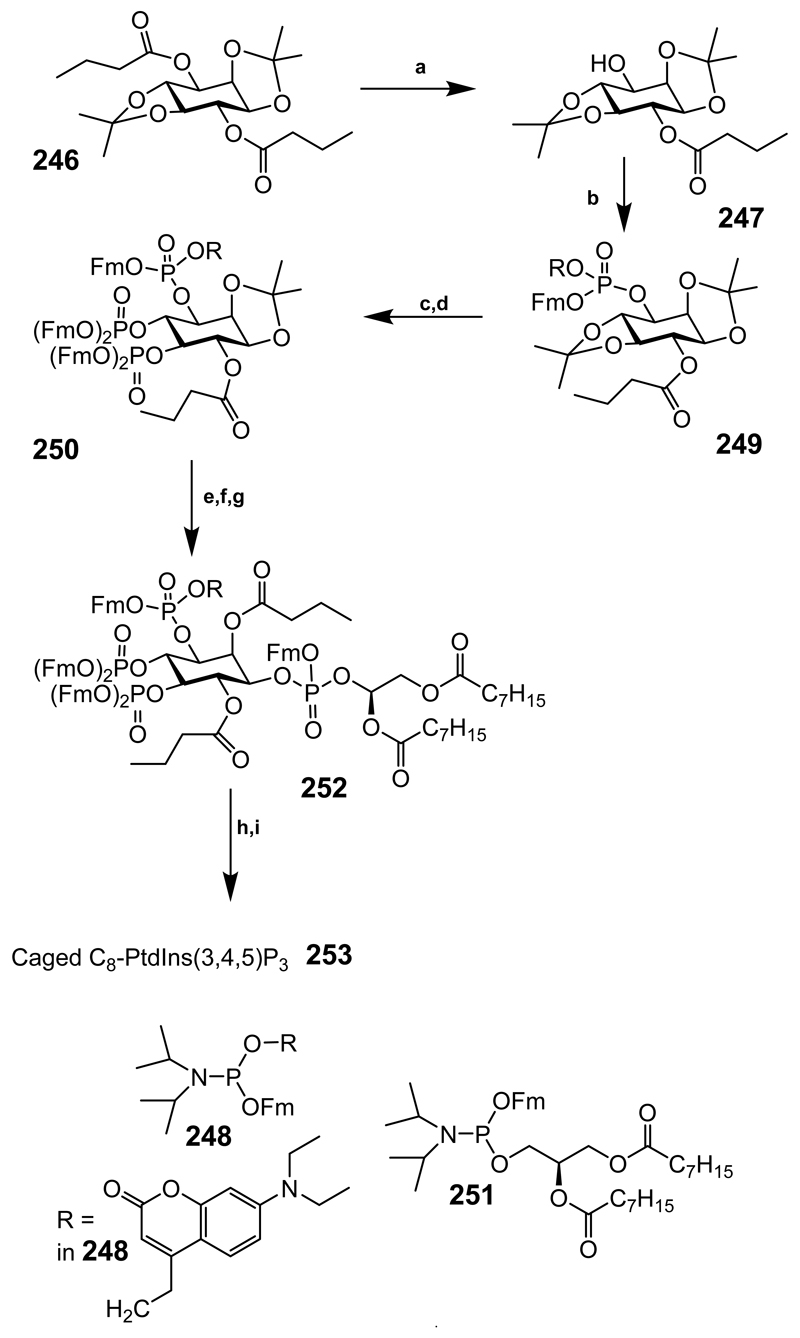
Scheme 33.
Reagents and conditions: (a) EtiPr2N-MeOH, (1:4), 36°C, 11 h, 91%; (b) Reagent 248, 4,5-dicyanoimidazole, CH2Cl2, 0°C, to rt, 20 min., then AcOOH, –18°C, to rt, 1 h, 70%; (c) Formic acid-CH2Cl2 (7:3), rt, 3.5 h; (d) Reagent 198, 4,5-dicyanoimidazole, CH2Cl2/MeCN, 4:1, rt, 1 h, then AcOOH, –18°C, to rt, 1.5 h, 48% (over 2 steps); (e) Formic acid/CH2Cl2, 95:5, 4.5 h; (f) 1,1-Dimethoxybutane, CH2Cl2, jandajel pyridinium trifluoroacetate, rt, 23 h, Dowex 50WX8, H+, 1 h; (g) Reagent 251 4,5-dicyanoimidazole, CH2Cl2-MeCN, rt, 30 min, then AcOOH, –18°C, to rt, 30 min, 46% (over 3 steps); (h) CH2Cl2, piperidine, rt, 1 h; (i) bromomethyl acetate, EtiPr2N, MeCN, rt, 10 h, 16% (over 2 steps).