Table 2.
Comparison of 1H NMR and ECD data and stereoconfiguration in angular tricyclic azaphilones.
| Compound | H-8 | H3-9 | C-7 | H-14 | ECD (nm) | |
|---|---|---|---|---|---|---|
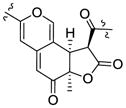
| Sassafrin A15 | 3.92 (12.4) | 1.60 | R | 3.67 (12.4) | +367, −321, +274 |
|
| Sassafrin B15 | 3.98 (12.4) | 1.59 | R | 3.68 (12.4) | +373, −322, +263 | |
| Longirostrerone C28 | 3.89 (12.9) | 1.42 | R | 4.14 (12.9) | +366, +332, −276 | |
|
| ||||||
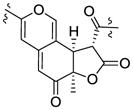
| Monascuskaolin18 | 3.86 (12.0) | 1.58 | 3.71 (12.0) |
|
||
| Biscogniazaphilone B17 | 3.86 (12.0) | 1.58 | 3.73 (12.0) | |||
| Berkchaetoazaphilone A (1) | 3.83 (12.2) | 1.55 | R | 3.73 (12.2) | +373, −330, +258 | |
|
| ||||||
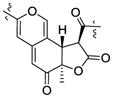
| Cohaerin G25 | 3.80 (12.5) | 1.39 | R | 4.08 (12.5) | +350, −272 |
|
| Cohaerin H25 | 3.90 (13.2) | 1.43 | R | 4.11 (12.9) | +350, −273 | |
| Monochaetin16 | 3.76 (12.8) | 1.36 | R | 4.07 (12.8) | ||
|
| ||||||
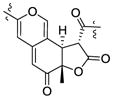
| Chermesinone B26 | 3.81 (12.9) | 1.36 | Sa | 4.09 (12.9) |
|
|
|
| ||||||
| Monapurone A27 | 3.31 | 1.28 | R | +369, −326, +258 |
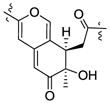
|
|
| Berkchaetoazaphilone C (5) | 3.35 | 1.28 | R | +363, −322, +253 | ||
| Helotialin A29 | 3.39 | 1.12 | R | +370, −324, −261 | ||
|
| ||||||
| Cohaerin I25 | 3.26 | 1.11 | R | +366, +319, −245 |
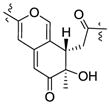
|
|
| Cohaerin K25 | 3.36 | 1.15 | R | +369, +319, −271 | ||
| Longirostrerone B28 | 3.34 | 1.14 | R | +370, +318, −247 | ||
