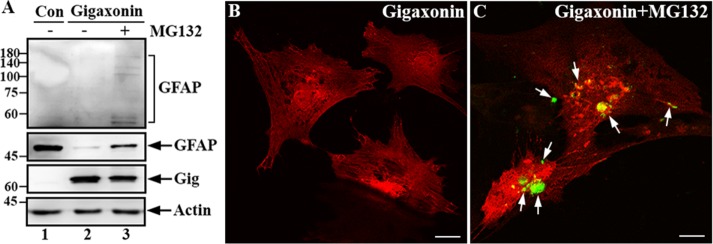
FIGURE 4:
Gigaxonin targeted GFAP for degradation through the proteasomal pathway. (A) Primary astrocytes were either mock infected (lane 1) or infected with lentiviruses containing gigaxonin (lanes 2 and 3). At 72 h after infection, gigaxonin-infected cells were treated with DMSO (lane 2) or 10 μM MG-132 (lane 3) for 12 h. Total lysates prepared from these cultures were analyzed by immunoblotting using antibodies to gigaxonin (Gig), GFAP, and actin, which was used as a loading control. Molecular mass markers (kilodaltons) are shown on the left. Note that increased exposure of the immunoblot was required to reveal the laddering of high–molecular weight GFAP (A, top, lane 3). Representative blots from three independent experiments. (B, C) Immunofluorescence showed the reappearance of GFAP. Primary astrocytes that had been cleared of GFAP by expression of gigaxonin for 72 h (B) were treated with MG-132 for 12 h (C). Cells were then double labeled with anti-GFAP (B and C, green channel) and anti-gigaxonin (B and C, red channel) antibodies. Merged images are shown. Note that GFAP reappeared as small aggregates (C, arrows) upon inhibition of the proteasome. Bar, 20 μm.