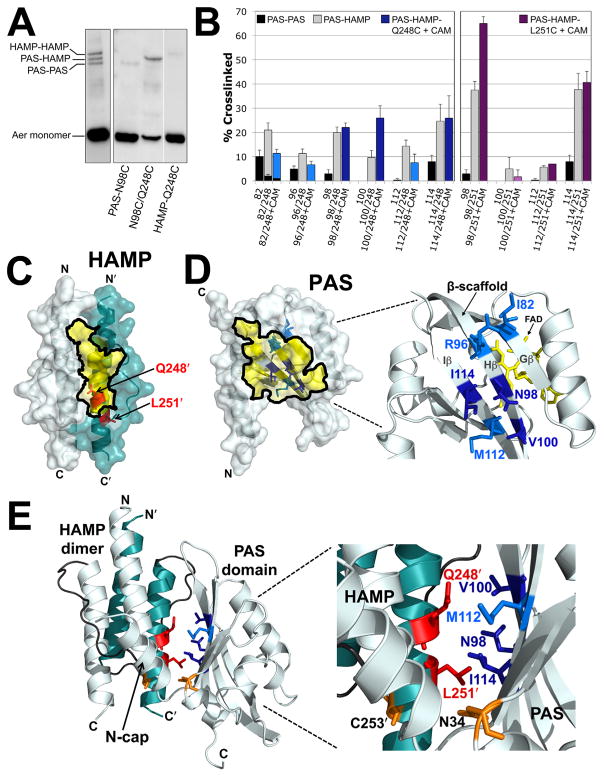
Fig. 5. Disulfide crosslinking between the Aer PAS and HAMP domains.
A. Representative examples comparing SDS-PAGE mobilities for Aer dimers crosslinked between PAS-PAS, PAS-HAMP and HAMP-HAMP. The left panel illustrates the separation of the three crosslinked bands for a di-Cys mutant with similar band densities at each position (Aer-I82C/A245C). The right panel illustrates the separation of these bands for a di-Cys mutant with preferential PAS-HAMP crosslinking (Aer-N98C/Q248C). In all cases, uncrosslinked monomer bands are near the bottom of the gels.
B. Extent of disulfide formation for PAS residues that preferentially crosslinked with HAMP-Q248C (left panel) and -L251C (right panel). PAS-PAS crosslinking is indicated by black fill, PAS-HAMP crosslinking by grey fill, PAS-Q248C crosslinking after treatment with chloramphenicol in blue fill and PAS-L251C crosslinking after treatment with chloramphenicol in purple fill. Dark blue or dark purple fill indicates that the extent of PAS-HAMP crosslinking did not significantly decrease after chloramphenicol treatment, whereas lighter blue or purple fill indicates a decreased extent of PAS-HAMP crosslinking after chloramphenicol treatment. HAMP-Q248C and -L251C controls routinely produced ≤ 1% dimers and are not shown. Error bars represent the standard deviation from multiple experiments.
C. HAMP homology model showing the location of HAMP-Q248 and -L251, which crosslinked with PAS, in relation to the region that was sequestered from PEG-mal (Fig. 3B, yellow shading).
D. PAS homology model showing the β-scaffold positions of the PAS residues that preferentially crosslinked with HAMP-Q248C in relation to the region that was sequestered from PEG-mal (Fig. 4D, yellow shading). Residue colours match those used in the left panel of B.
E. PAS-HAMP dimer model showing the proposed orientation of the PAS and HAMP domains based on PEGylation and PAS-HAMP crosslinking data. For clarity, the model includes the PAS domain from just one subunit (res. 5–119) and a HAMP dimer (monomers from both subunits, res. 204–258). The PAS model was manually oriented relative to the HAMP AS-2 helix of the cognate monomer to account for the crosslinking (B) and sequestration (C and D) data. Residue colours match those used in B, C and D. The locations of PAS-N34D and HAMP-C253R, which were previously identified as site-specific suppressors (Watts et al., 2004), are shown in orange. Abbreviation: CAM, chloramphenicol.