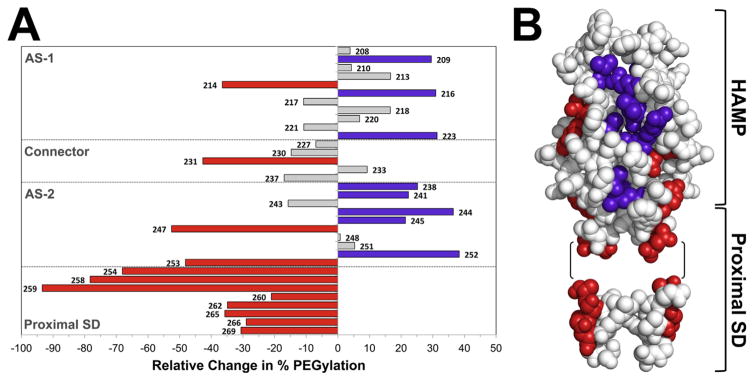
Fig. 6. Influence of the PAS kinase-on lesion, N85S, on the accessibility of residues in the HAMP and proximal signalling domains to PEG-mal.
A. Histogram showing the average percent change in PEGylation for 34 Cys substitutions in Aer-N85S. Bars projecting to the right of the origin denote residues that became more accessible in the presence of N85S (significant increases are coloured purple; P <0.05). Bars projecting to the left denote residues that became less accessible (significant decreases are coloured red; P <0.05). Residues in grey had statistically insignificant changes.
B. Location of residues from A that showed significant changes in accessibility (in the kinase-on state) when mapped onto models of the Aer-HAMP domain and part of the proximal signalling domain. The models include residues 204–258 and 262–269. Residues 259–261 were omitted because of the phase shift in this region. Residues 262–269 are modeled onto a 3.5 residue per turn coiled coil α-helix; the precise orientation of this region relative to HAMP is unknown.