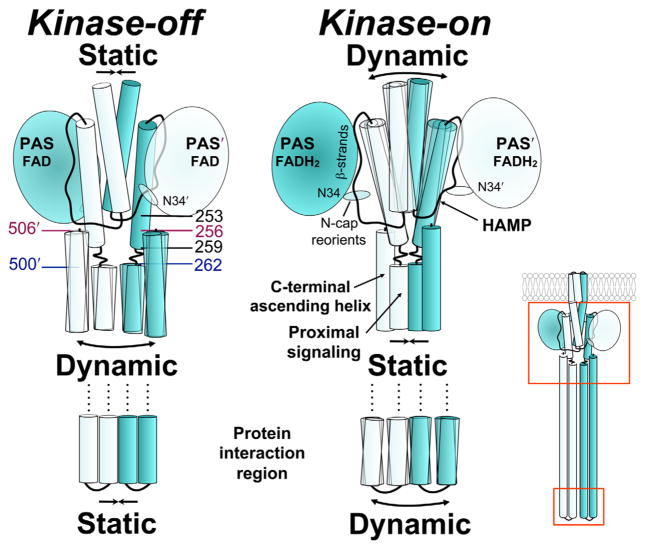
Fig. 8.
Working model of Aer showing the relationship between PAS and HAMP in the kinase-off and kinase-on states, based on current and previous data. Colours match those of Fig. 5. The reduction of PAS-bound FAD elicits a conformational change that decreases interaction between the PAS domain and the HAMP domain. The relative orientation between the N-cap and PAS core also changes, perhaps altering the stability of PAS-HAMP interactions. Decreased PAS-HAMP interactions are accompanied by tilting of the HAMP helices and a more relaxed, dynamic HAMP structure. The HAMP, proximal signalling domain and protein interaction regions are in opposition across contiguous boundaries such that a relaxed HAMP domain results in a static proximal signalling domain and a dynamic protein interaction region. Relevant residues: N34D, C253R, allele-specific suppressors; 259–262, phase shift; pairs 262/500 and 256/506 are highlighted to show their relative latitudes on descending and ascending helices. See Results and Discussion for details.