Abstract
Leishmaniasis is an important neglected tropical disease, affecting more than 12 million people worldwide. The available treatments are not well tolerated and present diverse side effects in patients, justifying the search for new therapeutic compounds. In the present study, the therapeutic potential and toxicity of ursolic acid (UA), isolated from the leaves of Baccharis uncinella C. DC. (Asteraceae), were evaluated in experimental visceral leishmaniasis. To evaluate the therapeutic potential of UA, hamsters infected with L. (L.) infantum were treated daily during 15 days with 1.0 or 2.0 mg UA/kg body weight, or with 5.0 mg amphotericin B/kg body weight by intraperitoneal route. Fifteen days after the last dose, the parasitism of the spleen and liver was stimated and the main histopathological alterations were recorded. The proliferation of splenic mononuclear cells was evaluated and IFN-γ, IL-4, and IL-10 gene expressions were analyzed in spleen fragments. The toxicity of UA and amphotericin B were evaluated in healthy golden hamsters by histological analysis and biochemical parameters. Animals treated with UA had less parasites in the spleen and liver when compared with the infected control group, and they also showed preservation of white and red pulps, which correlate with a high rate of proliferation of splenic mononuclear cells, IFN-γ mRNA and iNOS production. Moreover, animals treated with UA did not present alterations in the levels of AST, ALT, creatinine and urea. Taken together, these findings indicate that UA is an interesting natural compound that should be considered for the development of prototype drugs against visceral leishmaniasis.
Keywords: Ursolic acid, Leishmania (Leishmania) infantum, Therapeutic potential, Toxicity
Graphical abstract
Highlights
-
•
Animals treated with UA had less parasites in the spleen and liver.
-
•
Animals treated with UA showed histological preservation of spleen and liver.
-
•
Animals treated with UA up-regulated the expression of IFN-γ mRNA and iNOS production.
-
•
UA was not toxic for experimental animals.
1. Introduction
Leishmaniases are infectious parasitic diseases caused by protozoans belonging to the Trypanosomatidae family, Kinetoplastida order, Leishmania genus. These diseases affect humans, several wild and domestic mammal species, as well as invertebrates belonging to the Diptera order, Psychodidae family, Lutzomyia genus in the New World, as well as Phlebotomus genus in the Old World (WHO, 2010).
Leishmaniases consist of a complex of diseases with important clinical spectrum and epidemiological diversity. Depending on the infecting species and the intrinsic characteristics of the host (Lana et al., 2015), cutaneous leishmaniasis or visceral leishmaniasis (VL) can be clinically characterized. So far, VL has been recognized as the most serious clinical form of this group of diseases (Monge-Maillo and López-Vélez, 2013).
In the New World, the only species causing VL is L. (Leishmania) infantum (syn. L. (L) chagasi) (Silveira and Corbett, 2010), with an incidence of 1.9 cases per 100,000 inhabitants and a 90% mortality rate if not treated properly (Gomes et al., 2016). VL is considered a generalized chronic disease, the first symptom of visceralization being a frequent and relapsing low fever with two or three daily peaks. Fever is the most notable symptom due to its irregular or remitting feature (Van Griensven and Diro, 2012). Splenomegaly and hepatomegaly are also important clinical signs that persist during the course of the disease. The chronicity of VL is marked by progressive weight loss and general weakening, hence increasing the risk of acquiring secondary infections. It might progress quickly, though, leading the patient to cachexia or death within a few weeks or months (Van Griensven and Diro, 2012).
Although different groups have made efforts to characterize affordable and safe vaccines for human VL, they are currently still under characterization (Passero et al., 2012, Duthie et al., 2016). Thus, chemotherapy remains the only possible method that can be used to eliminate parasites from tissues. Antimonial and amphotericin B are the standard drugs used in human therapy (Rath et al., 2003). Pentavalent antimony has been used for more than seven decades, and nowadays it is still used as the first choice of treatment for all clinical forms of leishmaniasis (Tempone et al., 2011). While effective, patients treated with this drug present local and systemic side effects, such as nausea, vomiting, weakness, myalgia, abdominal pain, skin rash, liver and heart toxicities (McGwire and Satoskar, 2014). Moreover, some reports suggest that in the New World L. (Viannia) braziliensis, L. (V.) guyanensis and L. (L.) infantum have acquired increased resistance against antimonial drugs (Tessarollo et al., 2015, de Moura et al., 2016, Moreira et al., 2015, Monte-Neto et al., 2015).
In cases of unsuccessful treatment with antimonial or disease relapse, amphotericin B is chosen as the second-line drug, being effective against amastigote forms. It has, however, a number of side effects, including nephrotoxicity and cardiotoxicity, which limit its use. Besides resistance against amphotericin B have been suggested for some Leishmania species (Chattopadhyay and Jafurulla, 2011). In more detail, resistance development seems to be related with a replacement of ergosterol, the main target for this drug, by cholesta-5,7,24-trien-3-ol in the parasite membrane, an increase in the levels of MDR1 protein, and an upregulation in the cascade of the tryparedoxin pathway, among other functional changes, which all together make the parasite more resistant to amphotericin B (Purkait et al., 2012).
In view of the serious side effects of drugs commonly used in leishmaniasis chemotherapy and the emergence of drug-resistant parasites, it is urgent to search for new compounds that require few cycles of administration and that are more effective and less toxic to patients or animals. An interesting alternative for the discovery of new therapeutic agents for the treatment of leishmaniasis is prospecting natural products from different sources, such as plants, which possess a wide range of secondary metabolites, including triterpenes (Passero et al., 2014, Duarte et al., 2016).
Triterpenoids are the most representative group of phytochemicals, comprising more than 20,000 known compounds that can be classified into groups based on their structural skeletons, such as cycloartanes, dammaranes, euphanes, friedelanes, lanostanes, lupanes, oleananes, tirucallanes, and ursanes, among others (Hill and Connolly, 2012). The diversity of triterpenes is highly associated with their broad range of pharmacological effects, and different studies have already shown that these compounds present multispecies action against Leishmania sp. (Gnoatto et al., 2008, Bero et al., 2011, Begum et al., 2014). Recently, it was demonstrated that ursolic acid (UA) displayed activity against promastigote and amastigote forms of L. (L.) amazonensis, L. (V.) braziliensis, L. (L.) chagasi, and L. (L.) major (Passero et al., 2011, Begum et al., 2014, Yamamoto et al., 2014, Yamamoto et al., 2015), suggesting that UA presents multispectral action. In spite of that, few works have performed in vivo studies in order to evaluate the therapeutic potential of this triterpene and, more importantly, up to now there has not been any report analyzing the therapeutic action of this compound on VL, one of the most important medical conditions caused by Leishmania parasites.
Based on the multispecies action of UA in Leishmania parasites, and considering the absence of reports on the therapeutic potential of UA in experimental VL, the present work aimed to analyze the therapeutic effect of UA isolated from the leaves of the Brazilian plant Baccharis uncinella C. DC. (Asteraceae) using the experimental model of VL.
2. Material and methods
2.1. General experimental procedures
The 1H and 13C NMR spectra were recordedat 300 MHz and 75 MHz, respectively, in a Bruker Advance III spectrometer using DMSO-d6 (Sigma-Aldrich Co., St Louis, MO, USA) as solvent and internal standard. Silica gel (230–400 mesh; Merck & Co., Kenilworth, NJ, USA) and Sephadex LH-20 (Sigma-Aldrich Co.) were used for column chromatographic separation, while silica gel 60 PF254 (Merck & Co.) was used for analytical TLC (0.25 mm). High-performance liquid chromatography (HPLC) purification was performed in a Dionex Ultimate 3000 chromatograph, using a Luna Phenomenex RP-18 column (3 μm; 150 × 5 mm) and an ultraviolet (UV)-diode array detector (DAD). All chemicals employed were of analytical reagent grade. Amphotericin B was purchased from Cristalia (Brazil) and solubilized in sodium chloride 0.9% (w/v).
2.2. Plant material
The leaves of B. uncinella were collected in Campos do Jordão, São Paulo, Brazil, in June 2005. The plant was authenticated by Dr. Oriana A. Fávero and the voucher specimen was deposited at the Herbarium of the Prefeitura Municipal de São Paulo with the reference number PMSP8983.
2.3. Extraction and isolation of UA
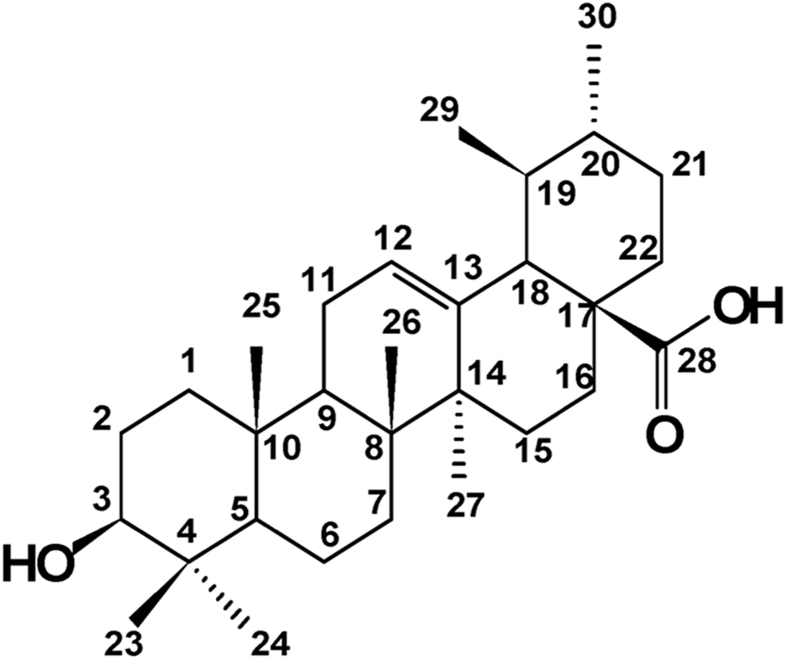
Dried and powdered leaves of B. uncinella (207 g) were exhaustively extracted using EtOH 95% at room temperature for 7 days. The crude extract (9.8 g), obtained after evaporation of solvent under reduced pressure, was dissolved in MeOH:H2O 7:3 (v/v) and partitioned using CH2Cl2. The obtained CH2Cl2 phase (5.3 g) was subjected to column chromatography over silica gel and eluted with different mixtures of n-hexane:EtOAc in gradient form to yield five groups (A–E). Group C (232 mg) was purified using semi-preparative RP-18 HPLC, eluted with MeOH:H2O 95:5 (flow rate: 5 mL/min; detector: λ = 218 nm) to afford 91 mg of a white amorphous solid. The isolated compound was identified as UA following NMR spectral analysis and comparison with the literature data (Olea and Roque, 1990), (UA: 99.8% purity, as determined by HPLC). 1H NMR (300 MHz, DMSO-d6) δH: 4.89 (br s, H-12), 4.06 (br s, H-3), 1.87 (d, J = 3.4 Hz, H-18), 1.68 (s, H-23), 1.61 (s, H-27), 1.52 (s, H-25), 1.31 (s, H-26), 0.80 (br s, H-30), 0.63 (br s, H-29), 0.58 (s, H-24). 13C NMR (75 MHz,n DMSO-d6) δC: 178.7 (C-28), 138.6 (C-13), 125.0 (C-12), 77.3 (C-3), 55.4 (C-5), 53.0 (C-18), 47.3 (C-9), 47.5 (C-17), 42.1 (C-14), 40.5 (C-8), 40.2 (C-19), 40.0 (C-10), 39.7 (C-22), 39.6 (C-7), 39.5 (C-21), 39.4 (C-23), 38.8 (C-15), 36.8 (C-1), 30.7 (C-4), 33.2 (C-20), 28.7 (C-2), 28.0 (C-29), 27.4 (C-16), 24.3 (C-27), 23.7 (C-11), 21.6 (C-30), 17.5 (C-6), 17.3 (C-26), 16.5 (C-25), 15.7 (C-24). The structure of UA is showed in Fig. 1.
Fig. 1.
The chemical structure of ursolic acid (UA) isolated from the leaves of B. uncinella.
2.4. Parasites and antigen production
L. infantum (syn. L. chagasi) parasite (MHOM/BR/72/46) was kindly provided by Prof. Dr. Fernando T. Silveira from the cryobank of the “Leishmaniasis Laboratory Prof. Dr. Ralph Laison”, Department of Parasitology, Evandro Chagas Institute, Ministry of Health, Belém, Pará State, Brazil. Species confirmation was accomplished using monoclonal antibodies and isoenzyme electrophoretic profiles at the Leishmaniasis Laboratory of the Evandro Chagas Institute. L. infantum was grown in M199 medium (Sigma-Aldrich Co.) supplemented with 10% fetal calf serum (FCS), 50,000 IU/mL penicillin, 50 μg/mL streptomycin, and 2% human urine at 25 °C. Stationary phase promastigotes were used throughout the entire study. To produce total antigen, L. infantum promastigotes were recovered by centrifugation at 1200 g for 10 min at 4 °C, washed three times with phosphate buffered saline (PBS), before resuspension in PBS with 1% protease inhibitors (Sigma-Aldrich Co.). Parasites were then lysed by successive freeze-thaw cycles.
2.5. Animals and ethical considerations
Golden hamsters (Mesocricetus auratus), 8 weeks old, were obtained from the Medical School of the University of São Paulo, Brazil. This study was carried out in strict accordance with the recommendations detailed in the Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation (http://www.cobea.org.br). The protocol was approved by the Ethics Committee of Animal Experiments of the Institutional Committee of Animal Care and Use at the Medical School of São Paulo University (CEP 259/13), and also by the Federal University of São Paulo (955422). For all experimental procedures, the animals were anaesthetized with tiopental (1 mg/200 μL).
2.6. Infection and experimental treatment
Twenty female golden hamsters were intraperitoneally infected with 2 × 107 promastigote forms of L. infantum, and 5 gold hamsters received only phosphate buffered saline (PBS) plus 1% DMSO under the same route (PBS group). Sixty days after infection, L. infantum-infected hamsters were divided into four groups: group 1 was injected with 1.0 mg of UA per kg of body weight (mg/kg); group 2 was injected with 2.0 mg/kg of UA; group 3 was injected with 5.0 mg/kg of amphotericin B (Corral et al., 2014), group 4 was injected with PBS plus 1% DMSO solution (infected control group). Group 5 was constituted by non-infected animals that received only the vehicle solution (PBS control). UA, amphotericin B, and the vehicle solution were injected intraperitoneally daily, once a day, over the course of 15 consecutive days. Fifteen days after the last injection, the animals were sacrificed in a CO2 chamber; their blood, spleen, kidney, lungs, heart, and liver were collected to analyze different parameters. Sera were collected and immediately stored at −80 °C and used for the evaluation of biochemical parameters. Before treatment, a few hamsters were euthanized in order to verify the presence of parasites in spleen and liver. Animals showed high parasitism and the organs presented macro- and microscopic alterations, as previously reported by our group (Duarte et al., 1988, Laurenti et al., 1990, Laurenti et al., 1996, Corbett et al., 1992, Corbett and Laurenti, 1998). UA was solubilized in DMSO and further PBS (never exceeding 1% DMSO); and amphotericin B was solubilized in sterile water for injection plus 1% DMSO.
2.7. Determination of parasite load
The parasite load was estimated using the quantitative limiting-dilution assay, as described by Campos et al. (2015), with minor modifications. Briefly, fragments of spleen and liver from the different groups were aseptically excised, weighted, and homogenized in M199 medium (Sigma-Aldrich Co.). The suspensions of organs were subjected to 12 serial dilutions with four replicate wells. The number of viable parasites was determined based on the highest dilution rate where promastigote forms could be observed after 15 days of cultivation at 25 °C.
In addition to the limiting-dilution assay, parasitism in the spleen and liver was evaluated by immunohistochemistry according to Laurenti and collaborators (Laurenti et al., 2014). Briefly, slides with histological sections were deparaffinized and hydrated. Antigenic recovery was developed in citric acid solution (10 mM, pH 6.0) for 3 min in a pressure cooker. Next, the slides were washed six times with 3% hydrogen peroxide (H2O2) to block endogenous peroxidase and to avoid nonspecific ionic binding; the sections were also incubated in a solution of powdered skim milk (10%), diluted in PBS, pH 7.4, at room temperature for 30 min. The immunolabeling reaction was performed with polyclonal mouse anti-Leishmania antibody at 1:1000 (produced in the Laboratory of Pathology of Infectious Diseases), diluted in PBS and 1% bovine serum albumin (BSA). This polyclonal antibody was raised against Leishmania infatum crude antigen in BALB/c mice and was standardizated to be used in immunohistochemistry. In order to detect the enzyme nitric oxide synthase 2 the polyclonal antibody anti-NOS2 (Santa Cruz, USA) was used at 1:200 in PBS plus 1% Bovine serum albumin, and add in histological section of spleen and liver for 60min, 37 °C. To develop the reaction, the LSAB kit (Dako Denmark A/S, Glostrup, Denmark) and diaminobenzidine (Sigma-Aldrich Co.) in PBS containing 3% hydrogen peroxide were used. Histological sections were counterstained in Harris's hematoxylin, dehydrated and mounted in resin with cover slides.
The main histopathological changes in the red and white pulps of the spleen, as well as in the portal regions and parenchyma of the liver were visualized in histological sections stained with hematoxylin and eosin (HE).
2.8. Analysis of cell proliferation
Spleens were individually homogenized in Roswell Park Memorial Institute (RPMI) 1640 medium and erythrocytes were lysed using lysis buffer (150 mM NH4Cl, 7 mM K2HCO3 and 0.01 mM EDTA). Cells were adjusted to 5 × 105 cells/well and cultured in sterile 96-well plates under specific stimulation with the total antigen (T-AG) of L. infantum promastigotes (10 μg/well) or under unspecific stimulation with concanavalin A (1 μg/well) as a positive control of proliferation. In addition, cells from all groups were cultured only with RPMI 1640 medium as negative controls. Plates were cultured in a humidified incubator at 37 °C under 5% CO2. Following 48 h of incubation, the plates were washed with PBS three times at 1000 rpm for 10 min, at 4 °C. Then, PrestoBlue reagent (Life Technologies, Carlsbad, CA, USA) was added to measure cellular proliferation (Sá-Nunes et al., 2009, Hamalainen-Laanaya and Orloff, 2012). After 2 h, fluorescence was read with the excitation and emission wavelengths at 570 nm and 620 nm, respectively. The fluorescent levels of negative controls were subtracted from all samples. The PBS control group (uninfected, untreated) did not proliferate under stimulation with the T-Ag of L. (L.) infantum.
2.9. Cytokine determination by real-time polymerase chain reaction (PCR)
RNA from spleen fragments (∼10 mg) was extracted using the commercial RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. cDNA was synthesized with the SuperScript® VILO™ cDNA Synthesis Kit (Life Technologies). Amplification conditions consisted of an initial denaturation phase at 95 °C for 10 min, followed by 40 amplification cycles consisting of 95 °C for 15 s, 61 °C for 90 s, and 72 °C for 30 s, using a thermocycler (Eppendorf, Hamburg, Germany). Prior to quantification, the efficiency of each reaction was verified using cDNA from spleens of healthy animal; it was always above 95%. Expression levels of genes of interest were normalized to β-actin (endogenous control). qPCR reaction was carried out using the GoTaq® qPCR Master Mix kit (Promega Corporation, Madison, WI, USA) and 25 nM of specific primers. The primer sequences were as follows (5′ to 3′): IFN-γ forward: GACAACCAGGCCATCC and reverse: CAAAACAGCACCGACT; IL-10 forward: TGGACAACATACTACTCACTG and reverse: GATGTCAAATTCATTCATGGC; IL-4 forward: CCACGGAGAAAGACCTCATCTG and reverse: GGGTCACCTCATGTTGGAAATAA; β-actin forward: TCCTGTGGCATCCACGAAACTACA and reverse: ACAGCACTGTGTTGGCATAGAGGT. Quantification results are expressed in fold changes of 2−ΔCt over the infected control group.
Prior to performing the qPCR, standard PCR reactions were performed to assess the specificity of the primers; one single amplification product of predicted size, according to Lafuse et al. (2013), was always obtained for such reactions.
2.10. Evaluation of the toxicity of UA
Healthy golden hamsters were divided into 4 groups containing 5 animals/group. Group 1 was treated intraperitoneally with 1.0 mg/kg of UA; group 2 was treated with 2.0 mg/kg of UA; group 3 was treated with 5.0 mg/kg of amphotericin B; and group 4 was injected with the vehicle solution (PBS). Animals were subjected to the same treatment described in 2.6. Five days after the last injection, animals were anaesthetized with thiopental and sacrificed by cardiac puncture. Sera were collected and immediately stored at −80 °C. Fragments of spleen, liver, kidney, lung, and heart were collected, processed histologically, and stained with HE. The biochemical parameters associated with hepatic and renal functions were evaluated through the quantification of seric alanine transaminase (ALT), aspartate aminotransferase (AST), urea (Sigma-Aldrich Co.) and creatinine (Labtest, Brazil).
3. Statistical analysis
The experiments were repeated four times and all parameters were assayed in triplicate; each experiment contained 25 golden hamsters. Experiments pertaining to toxicity contained 20 golden hamsters. The results were expressed by the arithmetic mean ± standard deviation. Statistical analyses were performed using GraphPad Prism 5.0, and the nonparametric Mann–Whitney U test was used to evaluate the differences between treated and infected control groups. Differences were considered statistically significant at a 5% significance level (P < 0.05).
4. Results
4.1. Determination of splenic and liver parasitism
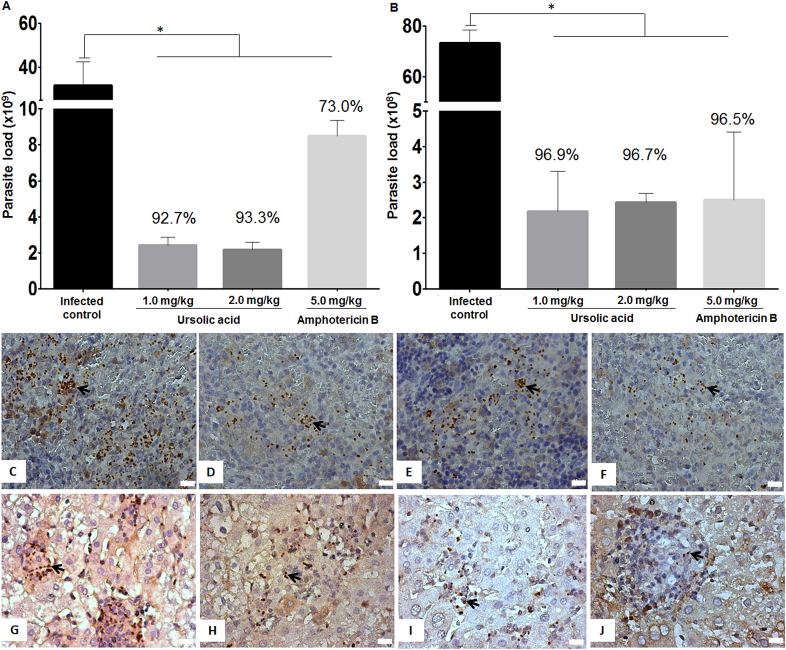
The parasite load in the spleen of infected hamsters treated with 1.0 or 2.0 mg/kg of UA, isolated from B. uncinella, was established at 2.3 × 109 (reduction of 92.7%) and 2.1 × 109 (reduction of 93.3%) parasite/g of spleen, respectively, when compared to the infected control group (31.5 × 109 parasite/g of spleen), as detailed in Fig. 2A (P < 0.05). In addition, amastigote reduction during UA treatment was evidenced by immunohistochemistry (Fig. 2D and E). Animals treated with 5.0 mg/kg of amphotericin B also showed reduction in splenic parasitism (8.5 × 109 parasite/g of spleen; reduction of 73%) when compared to the infected control group (P < 0.05) (Fig. 2A–F).
Fig. 2.
Parasite load in the spleen (A) and liver (B) of animals treated with UA or amphotericin B. Photomicrographs of histological sections stained by immunohistochemistry show amastigote forms (stained in dark brown) in the spleen (C, D, E and F) and liver (G, H, I and J) of the infected control group (C and G), as well as in the UA- (D, E, H, and I) or amphotericin B- (F and J) treated hamsters (400× magnification; scale bar: 10 μm). *P < 0.05 indicates statistical significance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Similarly, a significant reduction in parasitism was verified in the liver of animals treated with 1.0 mg/kg (2.2 × 108 parasite/g of liver; reduction of 96.9%) or 2.0 mg/kg (2.4 × 108 parasite/g of liver; reduction of 96.7%) of UA (P < 0.05) when compared with the infected control group, which presented liver parasitism of 72.9 × 108 parasite/g of liver (Fig. 2B, H and I). Animals treated with 5.0 mg/kg of amphotericin B presented a decrease in liver parasitism (2.5 × 108 parasite/g of liver; reduction of 96.5%) as well. Fig. 2J shows an immunolabeled histological section of liver from amphotericin B-treated animals to evidence amastigote forms.
4.2. Histopathological changes
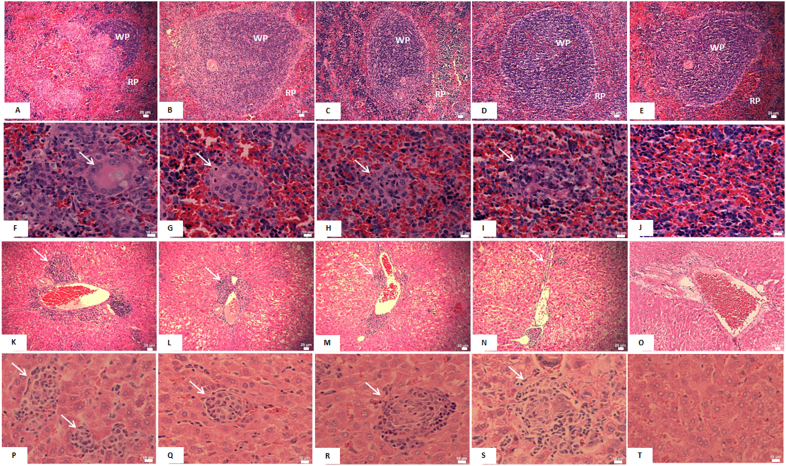
In the spleen of hamsters from the infected control group, a replacement of lymphoid follicles by infected macrophages was observed, suggesting immunosuppression caused by L. (L.) infantum (Fig. 3A). Furthermore, in the red pulp, proliferation of often-parasitized macrophages was observed and the presence of infected giant cells was also noted (Fig. 3F), indicating high disease severity evidenced by the histological sections stained by immunohistochemistry (Fig. 2C). Infected animals treated with 1.0 mg/kg and 2.0 mg/kg of UA showed preservation of the white pulp, suggesting a less severe condition and a better immune response (Fig. 3B–C). For these groups, nodules of macrophages were also detected in the red pulp, but they were fewer and exhibited less parasitism when compared to the infected control group (Fig. 3G–H). Animals treated with amphotericin B showed preservation of the white and red pulps (Fig. 3D) with few infected macrophages in the red pulp (Fig. 3I). Spleens from healthy animals exhibited white and red pulps with normal characteristics as shown in Fig. 3E–J.
Fig. 3.
Photomicrographs of histological sections of the spleen and liver from golden hamsters infected with L. (L.) infantum and treated with 1.0 mg/kg or 2.0 mg/kg of ursolic acid (UA) and 5.0 mg/kg of amphotericin B. Macrophagic invasion in the white pulp of the spleen (WP) was observed in the infected control group (A), and to a lesser degree in the animals treated with 1.0 mg/kg (B) or 2.0 mg/kg (C) of UA and amphotericin B (D). In the non-infected control group (E), no alterations were found. In the red pulp of the spleen (RP), macrophagic nodules (arrow) were found in all infected groups (F-I), whereas non-infected control animals did not present such alterations (J). Inflammation foci (arrow) were verified in liver portal areas of the infected control group (K), as well as of the UA- (L and M) or amphotericin B- (N) treated hamsters. Non-infected animals (O) showed normal histological characteristics. In the liver parenchyma, macrophagic granulomas (arrow) were observed in the infected control group (P) and in animals treated with UA (Q and R) or amphotericin B (S), while the non-infected control group did not exhibit such alterations (T). Spleen histological sections (A-J), 400× magnification; scale bars: 20 μm (A-E) and 10 μm (F-J). Liver histological sections (K-T), 100× magnification; scale bars: 20 μm (K-O) and 10 μm (P-T).
In the liver of all animal groups that were infected with L. (L.) infantum, independently of treatment, parasites were detected in portal areas that presented inflammatory foci characterized by the occurrence of lymphocytes and macrophages (white arrow in Fig. 3K–N). In addition, macrophage granulomas were also visualized in the liver parenchyma (white arrow in Fig. 3P–S). Treatment with UA and amphotericin B resulted, however, in lesser parasitism and fewer areas of portal inflammation.
4.3. Proliferation of splenic cells, expression of cytokines and nitric oxide synthase 2 (NOS2) immunostaining
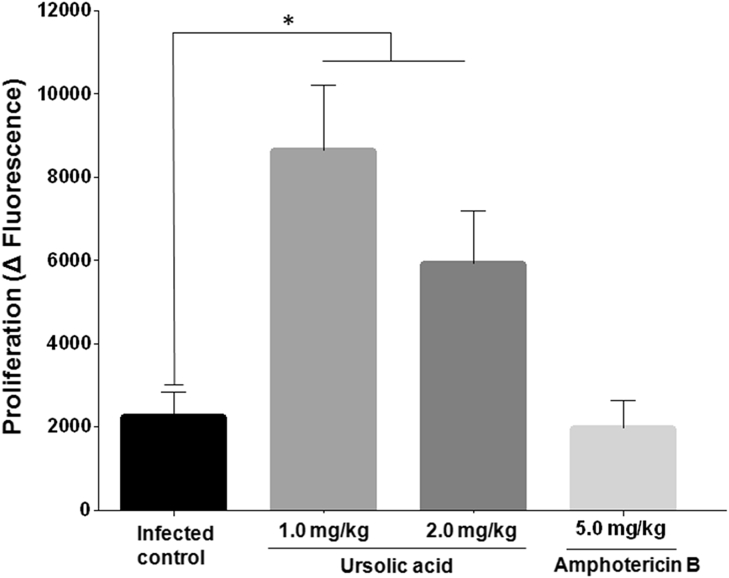
Splenic cells stimulated with T-Ag from animals treated with 1.0 mg/kg or 2.0 mg/kg of UA proliferated significantly more in comparison with the cells from the infected controls (P < 0.05). Cells from animals treated with 5.0 mg/kg of amphotericin B and stimulated with T-Ag did not proliferate (Fig. 4).
Fig. 4.
Proliferation of mononuclear cells in the spleen stimulated with 10 μg of total parasite antigen during 48 h *P < 0.05 indicates statistical significance when comparing the infected control group versus the treated groups.
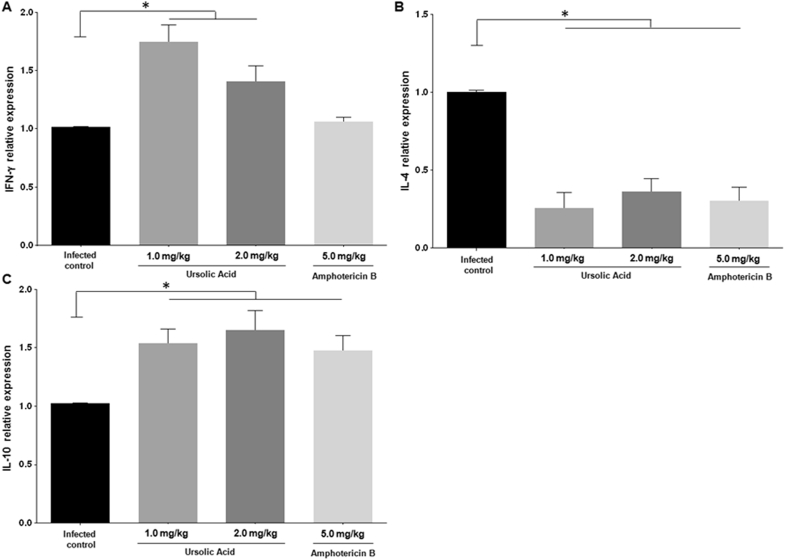
The spleen of infected golden hamsters treated with 1.0 mg/kg or 2.0 mg/kg of UA expressed higher levels of IFN-γ mRNA when compared to the infected control group (P < 0.05) (Fig. 5A). Infected animals treated with either concentration of UA or amphotericin B expressed significantly less IL-4 mRNA in the spleen than the infected control group (P < 0.05), as illustrated in Fig. 5B. Conversely, IL-10 gene expression was shown to be elevated in the spleens of animals treated with either of UA or amphotericin B (Fig. 5C).
Fig. 5.
Relative RNA expression of IFN-γ, IL-4, and IL-10. Cytokine gene expression was evaluated in the spleen of the treated and infected control groups. *P < 0.05 indicates significant differences when comparing the treated versus infected control group (control).
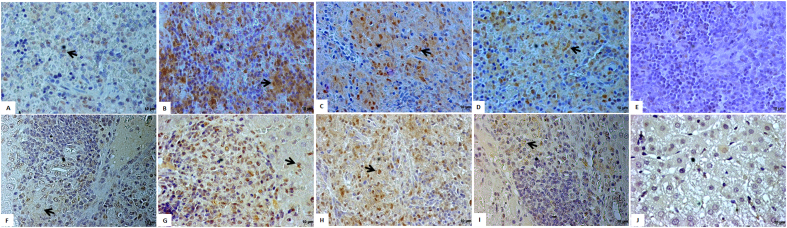
Infected hamsters treated with 1.0 mg/kg or 2.0 mg/kg of UA presented NOS2 positive areas diffusely detected throughout spleen histological sections, as illustrated in Fig. 6B–C. In addition, NOS2 positive areas could also be detected in the animals treated with amphotericin B, however it was in less amount and focally (Fig. 6D) when compared to histological section of animals treated with UA. The infected control group presented only basal positivity for NOS2 while the non-infected PBS control did not present positive areas for NOS2 enzyme (Fig. 6A–E, respectively).
Fig. 6.
Histological sections of spleen and liver of groups of hamsters were immunolabeled by immunohistochestry in order to detect NOS2 enzyme, the precursor of nitric oxide metabolite. Spleen sections of Infected Control, Animals treated with 1.0 mg/kg and 2.0 mg/kg of UA, animals treated with 5.0 mg/kg of amphotericin B and non-infected PBS control are represented in A, B, C, D and E, respectively. Similarly, histological sections of liver from Infected Control, Animals treated with 1.0 mg/kg and 2.0 mg/kg of UA, animals treated with 5.0 mg/kg of amphotericin B and non-infected PBS control are represented in F, G, H, I and J, respectively.
In the infected control group, few positive NOS2 cells were detected in inflammatory foci of periportal areas from the liver (Fig. 6F), while in infected animals treated with 1.0 and 2.0 mg/kg of UA NOS2 positive cells were detected in high number in the inflammatory areas of liver parenchyma (Fig. 6G–H, respectively). Infected animals treated with amphotericin B (Fig. 6I) presented few NOS2 positive cells, and the non-infected PBS control group did not show NOS2 positive cells (Fig. 6J).
4.4. Toxicity evaluation
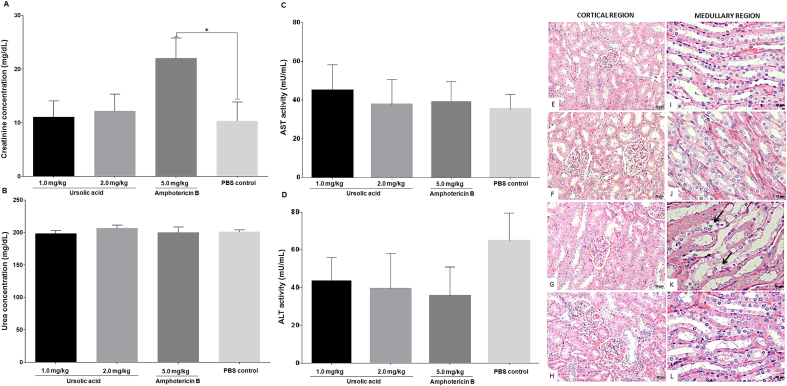
Healthy hamsters treated with 1.0 mg/kg and 2.0 mg/kg of UA or 5.0 mg/kg of amphotericin B did not show significant changes in the spleen, liver, lung, or heart (data not shown). Although UA did not alter the histology of the kidney, treatment with amphotericin B changes kidney medullary region. In this case, it was observed that the cuboidal epithelium of the distal tubule was necrotic (Fig. 7K). Histological changes were not detected in the cortical region of kidney of all animals analyzed (Fig. 7E and H).
Fig. 7.
Toxicity of UA and amphotericin B. Levels of serum creatinine (A), urea (B), AST (C) and ALT (D) were evaluated, and histological alterations were also recorded in the cortical and medullary regions of the kidney from healthy golden hamsters treated with 1.0 mg/kg (E and I) or 2.0 mg/kg of UA (F and J), or with 5.0 mg/kg of amphotericin B (G and K). The regions of the control group are shown in H and L. The arrows indicate necrotic areas (K). Cortical region: 100× magnification; scale bar: 20 μm. Medular region; 400× magnification; scale bar: 10 μm.
To confirm renal alterations induced by amphotericin B in golden hamsters, biochemical evaluations were carried out. In this regard, it was verified that animals treated with 1.0 mg/kg or 2.0 mg/kg of UA did not present seric alterations in creatinine or urea (Fig. 7A–B, respectively). In contrast, animals treated with 5.0 mg/kg of amphotericin B presented high levels of seric creatinine (P < 0.05) in comparison to the untreated control group (Fig. 7A). The levels of seric urea in all treated animals were similar to that of the control (Fig. 7B). Changes in the levels of either AST or ALT were not verified between treated and control animals (Fig. 7C–D, respectively).
5. Discussion
Currently, the main therapeutic arsenal available for treating leishmaniasis has been considered outdated. Moreover, patients face diverse side effects, and the drugs employed in present therapy can lead to the emergence of drug-resistant parasites. Therefore, it is urgent to characterize the leishmanicidal action of new compounds in vivo to increase the collection of effective prototype drugs. In this regard, UA seems to be an interesting target to develop new formulations to be used in human health, because different reports demonstrated its multivalent activities against pathological conditions. UA was demonstrated to be active against different tumor cell lines (Chuang et al., 2016, Aguiriano-Moser et al., 2015, Kim and Moon, 2015), its anti-tumoral effect being associated to apoptosis induction by intrinsic and extrinsic pathways of death (Li et al., 2014, Meng et al., 2015, Zhang et al., 2016, Villar et al., 2016).
In addition, in vivo experiments demonstrated the efficacy of UA, alone or in association with standard drugs, in colorectal and pancreatic tumors therapy (Shan et al., 2016, Prasad et al., 2016). Other studies also demonstrated the protective potential of UA in models of liver and endothelial cells injuries (Li et al., 2016), suggesting that the administration of high doses of UA trigger some antioxidant effects in experimental animals. Furthermore, UA was confirmed to possess antiprotozoal activities. Similarly to its effect on tumor cells, this triterpene induced programmed cell death in L. (L.) amazonensis, and presented therapeutic efficacy in the model of American Tegumentar Leishmaniasis (Yamamoto et al., 2015).
Despite the collection of related evidences, studies dealing with the antileishmanial effect of UA on experimental visceral leishmaniasis are rare. Thus, the present work aimed to characterize the therapeutic action of UA isolated from B. uncinella in experimental VL. In this regard, a reduction in splenic and liver parasitism was observed following treatment with UA, suggesting that the course of infection in treated animals was controlled when compared to the infected control group. Morever, in spleen, UA showed to be more effective than amphoterin B; in contrast, livers of animals treated with UA or amphotericin B showed similar parasitism, which was in both cases significantly less in comparison with the infected control group. Of note, in this study, amphotericin B did not tottaly cure infected hamster, possibly by the low number of injections, since it was not possible to continue the experimental treatment, because the infected control group was suffering from chronic and systemic infection that precluded the end of the experiment. Moreover, the therapeutic effect of UA impacted the histological architecture of the spleen and liver in treated animals. While the infected control group showed macrophagic invasion and disruption of the white and red pulps, suggesting immune suppression (Kaye et al., 2004, Rodrigues et al., 2016), animals treated with UA or amphotericin B demonstrated that the areas of lymphoid follicles, as well as the T-cell zones, remained intact, suggesting a better immune response pattern as confirmed by the reduction of viable parasites and by the decrease of parasitized areas.
The liver has been considered an acute site of infection by viscetropic species of Leishmania, as it features less damage than the spleen (Stanley and Engwerda, 2007). Still, the infected control group presented chronification of the inflammatory process in the portal regions, as well as granulomas in the liver parenchyma, while hamsters in the UA- and amphotericin B-treated groups exhibited a decrease in the inflammatory process with the presence of well-organized granulomas. According to several studies, the resolution of acute infection is associated with the development of inflammatory granulomas around Kupffer cells, although at the same time, the presence of granulomas can be considered a marker for parasite persistence (Engwerda et al., 2004, Stanley and Engwerda, 2007, Rodrigues et al., 2016). Giunchetti et al. (2008) showed that the liver of symptomatic dogs infected with L. infantum displayed portal inflammation and intralobular granulomas, among other histological changes, suggesting that these features could be associated with disease progression. Considering these findings, the UA triterpene showed a therapeutic effect on the liver of golden hamsters infected with L. infantum. Even though the increase in UA concentration did not improve its efficacy in the experimental model of visceral leishmaniasis, a fact that may have a direct association with UA bioavailability. Liao et al. (2005) as well as Chen et al. (2011) showed that after administration in animals UA could be rapidly detected in high levels in plasma, spleen and liver [55,56] – the main organs affected by L. infantum. However, it was quicky excreted, impacting the distribution or accumulation of this triterpene in the animal body; and causing a constant availability of UA independently of the administered dose. Therefore, similar number of parasites detected in the treatment with 1.0 or 2.0 mg/kg of UA may be related with the bioavailability of this compound.
Although the leishmanicidal action of UA (in vitro) was already evaluated (Passero et al., 2011, Yamamoto et al., 2015), its action in experimental models of leishmaniasis was poorly demonstrated until now. In this regard, Yamamoto and collaborators (Yamamoto et al., 2014) showed that UA treatment of L. (L.) amazonensis-infected BALB/c mice decreased skin parasitism, which was associated with the preservation of the epidermis and dermis as a possible consequence of IFN-γ production. Other studies showed that the astrakurkurone triterpene from the mushroom Astraeus hygrometricus was able to restrain parasitism in a murine model of VL, and this therapeutic action was associated with the upregulation of IFN-γ and IL-17 cytokines (Chen et al., 2011). Other terpenes were also shown to be active in VL, such as the clerodane diterpene 16α-hidroxicleroda clerodane-3,13Z-dien-15,16-olide from the leaves of Polyalthia longifolia, since hamsters treated with the diterpene exhibited splenic, liver, and bone marrow parasitism that was similar to that of animals treated with miltefosine (Misra et al., 2010). Additionally, an oleanane triterpenoid derivative (maesabalide III) isolated from Maesa balansae showed in vivo activity against L. donovani following administration of a single subcutaneous dose on either day 1 (prophylactic treatment) or day 28 (curative treatment) after infection (Maes et al., 2004). These data suggest that in addition to their therapeutic effects, some terpenes can also exert modulatory immune effects on animals (Yamamoto et al., 2014, Mallick et al., 2016).
Indeed, in the present study, it was demonstrated that golden hamsters infected with L. infantum and treated with UA exhibited preservation of important areas of the spleen, and that mononuclear cells stimulated with parasite T-Ag proliferated significantly more in comparison with the mononuclear cells of the infected control group and amphotericin B-treated animals. In VL, it is widely recognized that mononuclear cells have low, or even absent, proliferative potential to parasite antigens, indicating the immunosuppressive status of the cellular immune response (Fazzani et al., 2011). Therefore, the ability of mononuclear cells to proliferate under specific antigens suggests that the animals in the present study developed a beneficial immune response following treatment. Although animals treated with amphotericin B presented low parasitism and preservation of the white and red pulps, the mononuclear cells of these animals did not proliferate under specific antigens, a fact that point out to some specific immune suppression. However, this effect should not be understood as immunosuppression, since mononuclear cells of this group proliferated under stimulation with concanavalin A (data not shown). Previous studies also indicated that spleen cells from golden hamsters treated with free or encapsulated amphotericin B did not proliferate under specific stimuli (Dea-Ayuela et al., 2004, Dea-Ayuela et al., 2007), but such findings should not be considered as immunossuppresion, since concanavalin A-stimulated cells proliferated.
The dichotomy of the specific cellular immune response is one of the main factors that determine disease development or control, particularly because IFN-γ is an important marker of resistance (Lykens et al., 2010). On the contrary, IL-4 and IL-10 are recognized markers of susceptibility to infection (Osorio et al., 2014). In the present study, this dichotomy was evaluated by the relative expression levels of IFN-γ, IL-4, and IL-10 genes in the spleen. Infected hamsters treated with UA showed a polarized Th1 immune response that was related to protection (Kima and Soong, 2013). However, the increase in IL-10 expression verified in the UA- and amphotericin B-treated animals may regulate the inflammatory effect of IFN-γ. Studies have shown that although IL-10 may prevent tissue injuries caused by an exacerbated inflammatory response (Banchereau et al., 2012), this cytokine is also responsible for parasite maintenance at the site of infection, especially since IL-10 limits the generation of IFN-γ, as well as the microbicidal activity of macrophages (Belkaid et al., 2001), thus allowing parasites to survive inside host cells. Even in the group treated with amphotericin B, a drug used in leishmaniasis therapeutics, IL-10 exhibited increased gene expression and low amounts of IFN-γ mRNA. Moreover, parasites were visualized in histological sections and in the limiting-dilution assay, suggesting that the presence of IL-10 can be a crucial factor for maintaining tissue parasites, even in conditions that are adverse for them.
On the other hand, all treated groups presented decreased IL-4 expression, and although its role is not entirely clear in leishmaniasis, the IL-4 cytokine may be associated with pathogenic effects in experimental VL, as it was able to activate the STAT-6 transcription factor. This led to a strong expression of the gene encoding for L-arginase, an important enzyme that inhibits NO production (Osorio et al., 2014), which is essential for parasite elimination. Therefore, decreased expression of this cytokine following experimental treatments may be associated with a beneficial response against experimental VL. In fact, the infected control group expressed high amounts of IL-4 gene and produced only marginal levels of NOS2 in spleen or liver suggesting that IL-4 can be a factor associated with the pathogenesis of VL. Yet the spleen of UA-treated animals presented low IL-4 and high IFN-γ expression, inducing the Th1 immune response and macrophage activation by NOS2 mechanism, as verified in the spleen and liver, and culminating with parasite reduction. In histological section of the spleen from animals treated with amphotericine B, NOS2 positive areas were also detected, but the treatment with UA seems to induce a stronger reaction associated with the NOS2, the final enzyme responsible to trigger nitric oxide production. It is still important to note that the majority of immunological studies were carried out with qPCR because immunological reagents for hamster are rare.
Considering the therapeutic effect of UA in experimental VL, toxicity studies in golden hamsters were conducted. Histopatholocial analysis verified that the treated animals did not develop tissue injuries in the liver, spleen, heart, lung or kidney. Conversely, animals treated with amphotericin B displayed injuries in the medullary area of the kidney. In this case, tubular epithelial cells suffered an extensive process of necrosis. A study conducted by Berdichevski et al. (2006) demonstrated that patients treated with amphotericin B showed nephrotoxicity, which was characterized by a reduced glomerular filtration rate and tubular dysfunction. Furthermore, creatinine levels were higher and acute renal failure occurred in 31% of patients. In our study, the process of tubular necrosis found in animals treated with amphotericin B was associated with increased levels of serum creatinine, as already described by a previous study (Deray, 2002). Taken together, these findings demonstrated that UA is not a toxic drug for golden hamsters, while amphotericin B, although recognized as a leishmanicidal drug, can induce critical injuries. Moreover, a recent study demonstrated that UA had a nephroprotective effect by attenuating renal damage induced by toxic compounds (Pai et al., 2012), suggesting that this natural product has the potential to be considered as a prototype drug. Remarkably, UA triterpene was active in lower doses compared to the standard drug amphotericin B, and considering the molecular mass of both compounds, it is possible to assume that animals were treated with pharmaceutical dosage of UA, allowing its formulation as a medicament.
Different natural products had already been tested in viscerotropic Leishmania species, and the results had demonstrated that in some cases these natural compounds could be classified as promising antileishmanial drugs. Our present work evidenced that UA was an effective drug in the treatment of experimental VL without inducing toxicity; at the same time, it avoided immunosuppression by inducing a Th1 immune response leading to parasite elimination. Taken together, these findings support the conclusion that UA has an important leishmanicidal potential that is comparable to that of the standard drug, amphotericin B. UA can thus be considered an interesting candidate for further testing as a prototype drug for the treatment of VL.
Acknowledgments
The authors would like to thank the São Paulo Research Foundation (FAPESP) for their support [Grants 2013/16297-2, 2013/10133-8, 2015/11936-2 and 2016/00468-0] and HCFMUSP-LIM50. Authors also thank CNPq scientific research award to MDL and JHGL.
References
- Aguiriano-Moser V., Svejda B., Li Z.X., Sturm S., Stuppner H., Ingolic E., Höger H., Siegl V., Meier-Allard N., Sadjak A., Pfragner R. Ursolic acid from Trailliaedoxa gracilis induces apoptosis in medullary thyroid carcinoma cells. Mol. Med. Rep. 2015;12:5003–5011. doi: 10.3892/mmr.2015.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Pascual V., O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat. Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S., Ayub A., Qamar Zehra S., Shaheen Siddiqui B., Iqbal Choudhary M., Samreen Leishmanicidal triterpenes from Lantana Camara. Chem. Biodivers. 2014;11:709–718. doi: 10.1002/cbdv.201300151. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hoffmann K.F., Mendez S., Kamhawi S., Udey M.C., Wynn T.A. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevski R.H., Luis L.B., Crestana L., Manfro R.C. Amphotericin B-related nephrotoxicity in low-risk patients. Braz. J. Infect. Dis. 2006;10:94–99. doi: 10.1590/s1413-86702006000200005. [DOI] [PubMed] [Google Scholar]
- Bero J., Hannaert V., Chataigné G., Hérent M.F., Quetin-Laclercq J. In vitro antitrypanosomal and antileishmanial activity of plant used in Benin in traditional medicine and bio-guided fractionation of the most active extract. J. Ethnopharmacol. 2011;137:998–1002. doi: 10.1016/j.jep.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Campos B.L., Silva T.N., Ribeiro S.P., Carvalho K.I., Kallás E.G., Laurenti M.D. Analysis of iron superoxide dismutase-encoding DNA vaccine on the evolution of the Leishmania amazonensis experimental infection. Parasite Immunol. 2015;37:407–416. doi: 10.1111/pim.12206. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Jafurulla M. A novel mechanism for an old drug: amphotericin B in the treatment of visceral leishmaniasis. Biochem. Biophys. Res. Commun. 2011;416:7–12. doi: 10.1016/j.bbrc.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Chen Q., Luo S., Zhang Y., Chen Z. Development of a liquid chromatography–mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011;399:2877–2884. doi: 10.1007/s00216-011-4651-x. [DOI] [PubMed] [Google Scholar]
- Chuang W.L., Lin P.Y., Lin H.C., Chen Y.L. The apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by the PI3K/akt, p38 and JNK MAPK signaling pathways. Molecules. 2016;21:460. doi: 10.3390/molecules21040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett C.E.P., Laurenti M.D. Early detection of Leishmania (Leishmania) Chagasi in draining lymph node after subcutaneous inoculation in Hamsters. Parasitol. Int. Japao. 1998;47:307–310. [Google Scholar]
- Corbett C.E., Paes R.A., Laurenti M.D., Andrade Júnior H.F., Duarte M.I. Histopathology of lymphoid organs in experimental leishmaniasis. Int. J. Exp. Pathol. 1992;73:417–433. [PMC free article] [PubMed] [Google Scholar]
- Corral M.J., Serrano D.R., Moreno I., Torrado J.J., Domínguez M., Alunda J.M. Efficacy of low doses of amphotericin B plus allicin against experimental visceral leishmaniasis. J. Antimicrob. Chemother. 2014;69:3268–3274. doi: 10.1093/jac/dku290. [DOI] [PubMed] [Google Scholar]
- de Moura T.R., Santos M.L., Braz J.M., Santos L.F., Aragão M.T., de Oliveira F.A. Cross-resistance of Leishmania infantum isolates to nitric oxide from patients refractory to antimony treatment, and greater tolerance to antileishmanial responses by macrophages. Parasitol. Res. 2016;115:713–721. doi: 10.1007/s00436-015-4793-4. [DOI] [PubMed] [Google Scholar]
- Dea-Ayuela M.A., Rama-Iñiguez S., Sánchez-Brunete J.A., Torrado J.J., Alunda J.M., Bolás-Fernández F. Anti-leishmanial activity of a new formulation of amphotericin B. Trop. Med. Int. Health. 2004;9 doi: 10.1111/j.1365-3156.2004.01296.x. 981–790. [DOI] [PubMed] [Google Scholar]
- Dea-Ayuela M.A., Rama-Iñiguez S., Alunda J.M., Bolás-Fernandez F. Setting new immunobiological parameters in the hamster model of visceral leishmaniasis for in vivo testing of antileishmanial compounds. Vet. Res. Commun. 2007;31:703–717. doi: 10.1007/s11259-007-0040-5. [DOI] [PubMed] [Google Scholar]
- Deray G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002;1:37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- Duarte M.C., Tavares G.S., Valadares D.G., Lage D.P., Ribeiro T.G., Lage L.M. Antileishmanial activity and mechanism of action from a purified fraction of Zingiber officinalis Roscoe against Leishmania amazonensis. Exp. Parasitol. 2016;166:21–28. doi: 10.1016/j.exppara.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Duarte M.I., Laurenti M.D., Andrade Júnior H.F., Corbett C.E. Comparative study of the biological behaviour in hamster of two isolates of Leishmania characterized respectively as L. major-like and L. donovani. Rev. Inst. Med. Trop. 1988;30:21–27. doi: 10.1590/s0036-46651988000100004. [DOI] [PubMed] [Google Scholar]
- Duthie M.S., Favila M., Hofmeyer K.A., Tutterrow Y.L., Reed S.J., Laurance J.D. Strategic evaluation of vaccine candidate antigens for the prevention of Visceral Leishmaniasis. Vaccine. 2016;34:2779–2786. doi: 10.1016/j.vaccine.2016.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda C.R., Ato M., Kaye P.M. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends. Parasitol. 2004;20:524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Fazzani C., Guedes P.A., Sena A., Souza E.B., Goto H., Lindoso J.A.L. Dynamics of immunosuppression in hamsters with experimental visceral leishmaniasis. Braz. J. Med. Biol. Res. 2011;44:666–670. doi: 10.1590/s0100-879x2011007500062. [DOI] [PubMed] [Google Scholar]
- Giunchetti R.C., Mayrink W., Carneiro C.M., Corrêa-Oliveira R., Martins-Filho O.A., Marques M.J. Histopathological and immunohistochemical investigations of the hepatic compartment associated with parasitism and serum biochemical changes in canine visceral leishmaniasis. Res. Vet. Sci. 2008;84:269–777. doi: 10.1016/j.rvsc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Gnoatto S.C., Dalla Vechia L., Lencina C.L., Dassonville-Klimpt A., Da Nascimento S., Mossalayi D. Synthesis and preliminary evaluation of new ursolic and oleanolic acids derivatives as antileishmanial agents. J. Enzyme Inhib. Med. Chem. 2008;23:604–610. doi: 10.1080/14756360802204870. [DOI] [PubMed] [Google Scholar]
- Gomes A.P., Miguel P.S.B., Vitorino R.R., Correia M.D.L., Souza R.F.D., Freitas R.B. Visceral Leishmaniasis: a brazilian perspective. J. Trop. Dis. 2016;4:202. [Google Scholar]
- Hamalainen-Laanaya H.K., Orloff M.S. Analysis of cell viability using time-dependent increase in fluorescence intensity. Anal. Biochem. 2012;429:32–38. doi: 10.1016/j.ab.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Hill R.A., Connolly J.D. Triterpenoids, natural product. Reports. 2012;29:780–818. doi: 10.1039/c2np20027a. [DOI] [PubMed] [Google Scholar]
- Kaye P.M., Svensson M., Ato M., Maroof A., Polley R., Stager S. The immunopathology of experimental visceral leishmaniasis. Immunol. Rev. 2004;201:239–253. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- Kim E.S., Moon A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015;9:897–902. doi: 10.3892/ol.2014.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kima P.E., Soong L. Interferon gamma in Leishmaniasis. Front. Immunol. 2013;4:156. doi: 10.3389/fimmu.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuse W.P., Story R., Mahylis J., Gupta G., Varikuti S., Steinkamp H. Leishmania donovani infection induces anemia in hamsters by differentially altering erythropoiesis in bone marrow and spleen. PLoS One. 2013;8:e59509. doi: 10.1371/journal.pone.0059509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana R.S., Michalsky É.M., Fortes-Dias C.L., França-Silva J.C., Lara-Silva F., de O., Lima A.C. Phlebotomine sand fly fauna and leishmania infection in the vicinity of the Serra do Cipó National Park, a natural Brazilian heritage site. Biomed. Res. Int. 2015:385493. doi: 10.1155/2015/385493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti M.D., Corbett C.E.P., Sotto M.N., Sinhorini I.L., Goto H. The role of complement in the acute inflammatory process in the skin and in the host-parasite interaction in Hamsters inoculated with Leishmania (Leishmania) Chagasi. Int. J. Exp. Pathol. 1996;77:15–24. doi: 10.1046/j.1365-2613.1996.958096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti M.D., Passero L.F., Tomokane T.Y., Francesquini F., de C., Rocha M.C., Gomes C.M. Dynamic of the cellular immune response at the dermal site of Leishmania (L.) amazonensis and Leishmania (V.) braziliensis infection in Sapajus apella primate. Biomed. Res. Int. 2014 doi: 10.1155/2014/134236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti M.D., Sotto M.N., Corbett C.E., da Matta V.L., Duarte M.I. Experimental visceral leishmaniasis: sequential events of granuloma formation at subcutaneous inoculation site. Int. J. Exp. Pathol. 1990;71:791–797. [PMC free article] [PubMed] [Google Scholar]
- Li D., Ren D., Luo Y., Yang X. Protective effects of ursolic acid against hepatotoxicity and endothelial dysfunction in mice with chronic high choline diet consumption. Chem. Biol. Interact. 2016;258:102–107. doi: 10.1016/j.cbi.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Li Y., Lu X., Qi H., Li X., Xiao X., Gao J. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and suppression of ERK1/2 MAPK in HeLa cells. J. Pharmacol. Sci. 2014;125:202–210. doi: 10.1254/jphs.14017fp. [DOI] [PubMed] [Google Scholar]
- Liao Q., Yang W., JIA Y., Chen X., Gao Q., Bi K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract. J. Pharm. Soc. Jpn. 2005;125:509–515. doi: 10.1248/yakushi.125.509. [DOI] [PubMed] [Google Scholar]
- Lykens J.E., Terrell C.E., Zoller E.E., Divanović S., Trompette A., Karp C.L. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J. Immunol. 2010;184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L., Germonprez N., Quirijnen L., Van Puyvelde L., Cos P., Vanden Berghe D. Comparative activities of the triterpene saponin maesabalide III and liposomal amphotericin B (AmBisome) against Leishmania donovani in hamsters. Antimicrob. Agents Chemother. 2004;48:2056–2060. doi: 10.1128/AAC.48.6.2056-2060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick S., Dutta A., Chaudhuri A., Mukherjee D., Dey S., Halder S. Successful therapy of murine visceral Leishmaniasis with astrakurkurone, a triterpene isolated from the mushroom Astraeus hygrometricus, involves the induction of protective cell-mediated immunity and TLR9. Antimicrob. Agents Chemother. 2016;60:2696–2708. doi: 10.1128/AAC.01943-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwire B.S., Satoskar A.R. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107:7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Lin Z.M., Ge N., Zhang D.L., Huang J., Kong F. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/akt/mTOR pathway. Am. J. Chin. Med. 2015;43:1471–1486. doi: 10.1142/S0192415X15500834. [DOI] [PubMed] [Google Scholar]
- Misra P., Sashidhara K.V., Singh S.P., Kumar A., Gupta R., Chaudhaery S.S. 16alpha-Hydroxycleroda-3,13(14)Z-dien-15,16-olide from Polyalthia longifolia: a safe and orally active antileishmanial agent. Br. J. Pharmacol. 2010;159:1143–1150. doi: 10.1111/j.1476-5381.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge-Maillo B., López-Vélez R. Therapeutic options for visceral leishmaniasis. Drugs. 2013;73:1863–1888. doi: 10.1007/s40265-013-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Neto R., Laffitte M.C., Leprohon P., Reis P., Frézard F., Ouellette M. Intrachromosomal amplification, locus deletion and point mutation in the aquaglyceroporin AQP1 gene in antimony resistant Leishmania (Viannia) guyanensis. PLoS Negl. Trop. Dis. 2015;9:e0003476. doi: 10.1371/journal.pntd.0003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D., de S., Pescher P., Laurent C., Lenormand P., Späth G.F., Murta S.M. Phosphoproteomic analysis of wild-type and antimony-resistant Leishmania braziliensis lines by 2D-DIGE technology. Proteomics. 2015;15:2999–3019. doi: 10.1002/pmic.201400611. [DOI] [PubMed] [Google Scholar]
- Olea R.S.G., Roque N. Análise de misturas de triterpenos por RMN de 13C. Quím. Nova. 1990;13:278. [Google Scholar]
- Osorio E.Y., Travi B.L., da Cruz A.M., Saldarriaga O.A., Medina A.A., Melby P.C. Growth factor and Th2 cytokine signaling pathways converge at STAT6 to promote arginase expression in progressive experimental visceral leishmaniasis. PLoS Pathog. 2014;10:e1004165. doi: 10.1371/journal.ppat.1004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai P.G., Chamari Nawarathna S., Kulkarni A., Habeeba U., Reddy C.S., Teerthanath S. Nephroprotective effect of ursolic Acid in a murine model of gentamicin-induced renal damage. ISRN Pharmacol. 2012;2012:410902. doi: 10.5402/2012/410902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passero L.F., Bonfim-Melo A., Corbett C.E., Laurenti M.D., Toyama M.H., de Toyama D.O. Anti-leishmanial effects of purified compounds from aerial parts of Baccharis uncinella C.DC. (Asteraceae) Parasitol. Res. 2011;108:529–536. doi: 10.1007/s00436-010-2091-8. [DOI] [PubMed] [Google Scholar]
- Passero L.F., Laurenti M.D., Santos-Gomes G., Soares Campos B.L., Sartorelli P., Lago J.H. Plants used in traditional medicine: extracts and secondary metabolites exhibiting antileishmanial activity. Curr. Clin. Pharmacol. 2014;9:187–204. doi: 10.2174/1574884709999140606161413. [DOI] [PubMed] [Google Scholar]
- Passero L.F., Marques C., Vale-Gato I., Corbett C.E., Laurenti M.D., Santos-Gomes G. Analysis of the protective potential of antigens released by Leishmania (Viannia) shawi promastigotes. Arch. Dermatol. Res. 2012;304:47–55. doi: 10.1007/s00403-011-1171-7. [DOI] [PubMed] [Google Scholar]
- Prasad S., Yadav V.R., Sung B., Gupta S.C., Tyagi A.K., Aggarwal B.B. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget. 2016;7:13182–13916. doi: 10.18632/oncotarget.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkait B., Kumar A., Nandi N., Sardar A.H., Das S., Kumar S. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012;56:1031–1041. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S., Trivelin L.A., Imbrunito T.R., Tomazela D.M., Jesús M.N., Marzal P.C. Antimonials employed in the treatment of leishmaniaisis: the state of the art. Quím. Nova. 2003;26:550–555. [Google Scholar]
- Rodrigues V., Cordeiro-da-Silva A., Laforge M., Silvestre R., Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors. 2016;9:118. doi: 10.1186/s13071-016-1412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Nunes A., Bafica A., Antonelli L.R., Choi E.Y., Francischetti I.M., Andersen J.F. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J. Immunol. 2009;182:7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Xuan Y., Zhang Q., Zhu C., Liu Z., Zhang S. Ursolic acid synergistically enhances the therapeutic effects of oxaliplatin in colorectal cancer. Protein. Cell. 2016;7:571–585. doi: 10.1007/s13238-016-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley A.C., Engwerda C.R. Balancing immunity and pathology in visceral leishmaniasis. Immunol. Cell. Biol. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Corbett C.E.P. Leishmania chagasi Cunha & Chagas, 1937: nativa ou introduzida? Uma breve revisão. Ver. Pan-Amaz Saude. 2010;1:143–147. [Google Scholar]
- Tempone A.G., Oliveira C.M., Berlinck R.G.S. Current Approaches to discover marine antileishmanial natural products. Planta Medica. 2011;77:572–585. doi: 10.1055/s-0030-1250663. [DOI] [PubMed] [Google Scholar]
- Tessarollo N.G., Andrade J.M., Moreira D.S., Murta S.M. Functional analysis of iron superoxide dismutase-A in wild-type and antimony-resistant Leishmania braziliensis and Leishmania infantum lines. Parasitol. Int. 2015;64:125–129. doi: 10.1016/j.parint.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Van Griensven J., Diro E. Visceral leishmaniasis. Infect. Dis. Clin. North Am. 2012;26:309–322. doi: 10.1016/j.idc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Villar V.H., Vögler O., Barceló F., Martín-Broto J., Martínez-Serra J., Ruiz-Gutiérrez V. Down-regulation of AKT signalling by ursolic acid induces intrinsic apoptosis and sensitization to doxorubicin in soft tissue sarcoma. PLoS One. 2016;11:e0155946. doi: 10.1371/journal.pone.0155946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2010. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniasis. [Google Scholar]
- Yamamoto E.S., Campos B.L., Jesus J.A., Laurenti M.D., Ribeiro S.P., Kallás E.G. The effect of ursolic acid on Leishmania (Leishmania) amazonensis is related to programed cell death and presents therapeutic potential in experimental cutaneous leishmaniasis. PLoS One. 2015;10:e0144946. doi: 10.1371/journal.pone.0144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E.S., Campos B.L., Laurenti M.D., Lago J.H., Grecco S., dos S., Corbett C.E. Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mice. Parasitol. Res. 2014;113:333–339. doi: 10.1007/s00436-013-3659-x. [DOI] [PubMed] [Google Scholar]
- Zhang R.X., Li Y., Tian D.D., Liu Y., Nian W., Zou X., Chen Q.Z., Zhou L.Y., Deng Z.L. He BC. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int. J. Oncol. 2016;49:1973–1982. doi: 10.3892/ijo.2016.3701. [DOI] [PubMed] [Google Scholar]