Abstract
Nutrition transition to high energy-dense foods has been implicated as the major causes of diet related diseases. Plantain-based dough meals supplemented with soybean cake and cassava fibre were developed by combining them in different proportions using response surface methodology. The flour blends were analyzed for the nutritional composition while the glycaemic index, antidiabetic potentials and protein digestibility of the dough meals were determined in wistar rats. The nutritional and essential amino acid contents of the flour blends were comparable to that of cerolina (a commercially available food product commonly recommended for diabetic patients). The rats fed with the formulated dough meals had lower glycaemic index and glycaemic load, and the blood glucose was significantly reduced compared to cerolina and metformin (a synthetic antidiabetic drug). All the plantain-based dough meals were comparable to cerolina and metformin in terms of nutritional quality and blood glycaemic control activities, respectively. Hence, the formulated plantain-based dough meals have potential to be used for the prevention and management of diabetes mellitus.
Keywords: Plantain-based meals, Glycaemic index, Functional food, Type-2 diabetes
Introduction
Diabetes mellitus is a chronic metabolic disorder that is characterized by hyperglycemia, which results from insufficient or inefficient insulin secretion in the body, and it is associated with impaired carbohydrate, fat and protein metabolism. Type-2 diabetes, a non-insulin dependent diabetes mellitus, is the leading cause of death and disability, with over 285 million people living with diabetes worldwide (Shaw et al. 2010). The prevalence of Type 2 diabetes in developing countries is attributed to the nutrition transition and lifestyle changes from traditional diets, which are high in plant-based foods like grains, legumes and fruits/vegetables to more Western pattern diets that are high in sugars, fat, and animal-source foods (Popkin 2002; Kapoor and Anand 2002; Sun et al. 2010). This has also led to high prevalence of chronic and degenerative diseases.
The medicinal values of plant-based foods have assumed a more important dimension in recent years owing largely to the benefits that is associated with their nutrients and non-nutrients compositions (Eleazu et al. 2010). Cereals, grains and fruits/vegetables have substantial benefits in preventing and treating chronic diseases.
Plantain (Musa paradisiaca) is a giant perennial herb cultivated in many tropics and subtropical countries of the world. In Nigeria, it is the third most important staple after yam and cassava. It is a rich source of carbohydrates, iron, potassium and vitamin A, but low in fat and protein (Odenigbo et al. 2013). Plantain can be consumed in the ripe or unripe forms as well as boiled, fried, roasted, or when processed into flour and used to prepare local dough meal known as ‘amala’ (Idowu et al. 1996). It has remained the ultimate source of nutrients and non-nutrients that are beneficial to human in many parts of developing countries, and they are used for socio-cultural, diabolic, nutritional and therapeutic purposes (Eleazu et al. 2010).
Soybean is an important source of vegetable protein in many parts of developed and developing countries and it is utilized in various food productions such as tofu (soybean curd), miso (fermented soybean paste) and soy-ogi. The increased acceptance of soybeans and their products is primarily associated with the high nutritional quality especially with respect to protein and amino acids, good functional properties in food applications, high nutritional value, availability and low cost (Gandhi 2009).
Cassava is an important staple food and cash crop in Sub-Saharan Africa (Agwu and Anyaeche 2007). It is the cheapest and most affordable source of calories for the majority of people living in developing countries.
Quite a number of studies have utilized plantain, soybean and maize in the production of foods (Osundahunsi 2006; Abiose et al. 2015) but there is dearth of information on the combinations of plantain flour, defatted soybean cake and cassava fiber. In this study, plantain flour, defatted soybean and cassava fiber were used to formulate functional dough meals which were analyzed for their potentials to be used in the prevention and treatment of diabetes.
Materials and methods
Sources of food materials and wistar rats
Freshly harvested matured unripe plantain (Musa AAB) was purchased from Ogbese Market, Akure, Cassava fibre was obtained from Matna Food Company, Owo, (company processing cassava tubers into starch) and Soybean cake was obtained from JOF Ideal Family Farms, Owo, (company processing soybean to vegetable oil) all in Ondo State, Nigeria. The wistar rats were obtained from Central Animal House, College of Medicine, University of Ibadan, Ibadan, Nigeria. The study protocol was approved by the Ethical Committee for Laboratory Animals of the School of Agriculture and Agricultural Technology, Federal University of Technology Akure, Nigeria.
Preparation of flour samples
After the preliminary cleaning and washing, the unripe plantain, Soy cake is defatted soybean meal and cassava fibre were oven-dried at 70 °C for 8, 2 and 8 h, respectively. The dried samples were separately milled using Philips laboratory blender (HR2811 model; Hong Kong) and sieved using sieve number 60 (British Standard) to obtain the various flours which were then packed in plastic container, sealed and stored at room temperature (~27 °C) for further analyses.
Formulations of plantain-based functional dough meal
The optimum combination of plantain flour, soybean cake and cassava fibre in the blended flour samples were determined using Response Surface Methodology (RSM). (Gen Stat Discovery, Edition 4). RSM statistical package was used to generate proportion of flour samples in the formulation with references to fiber (10 %), carbohydrate (55–65 %) and protein (20 %) requirements for diabetic patients (Franz et al. 1994) as follows: PSC-1 (19.0 % defatted soybean cake, 5.0 % cassava fiber and 76.0 % plantain flour), PSC-2 (15.0 % defatted soybean cake, 8.0 % cassava fiber and 77.0 % plantain flour) and PSC-3 (25.0 % defatted soybean cake, 8.0 % cassava fiber and 67.0 % plantain flour).
Chemical compositions plantain-based functional dough meal
Determination of proximate composition
The proximate composition was determined according to AOAC (2005). Moisture content was determined in a hot-air circulating oven (Gallenkamp). Total ash was determined by incineration (550 °C) of known weights of the samples in a muffle furnace (Hotbox oven, Gallenkamp, UK, size 3). Crude fat was determined by exhaustively extracting a known weight of sample in petroleum ether. Protein content (gN × 6.25) was determined by the micro-Kjeldahl method. Crude fiber was determined after digestion and carbohydrate content was determined by difference. The gross energy value of the food samples were estimated [in kJ/100 g] by using this formula—Gross energy (kJ/100 g dry matter) = (protein × 16.7) + (lipid × 37.7) + (carbohydrates × 16.7).
Determination of amino acid profile
The amino acid compositions of the blended flour samples were determined as described by Glew et al. (2005) with slight modification while the tryptophan content was determined in a separate analysis as described by Buzzigoli et al. (1990).
Determination of the antinutritional factors
Oxalate determination
Oxalate was determined according to Day and Underwood (1986) procedure. The sample was weighed (1.0 g), 75 ml of 15 N H2SO4 was added, stirred intermittently with a magnetic stirrer for 1 h and filtered using Whatman No 1 filter paper. About 25 ml of the filtrate was collected and titrated against 0.1 N KMnO4 solutions till a faint pink colour appeared that persisted for 30 s.
Phytate determination
Phytate concentration was determined through phytic acid determination using the methods described by Lolas and Markakis (1975). The sample was weighed (2 g) into 250 ml conical flask, 100 ml of 2 % conc. HCl was added in the conical flask to soak the samples for 3 h, the sample mixture was filtered through a double layer filter paper, 50 ml of the sample filtrate was placed in a 250 ml beaker and 100 ml of distilled water was added and vortex for proper mixing. To the filtrate, 10 ml of 0.3 % ammonium thiocyanate solution was added as indicator and the filtrate solution was titrated with standard iron chloride solution which contained 1.95 mg iron/ml and the end point was signified by brownish-yellow colour that persisted for 5 min and the percentage phytic acid was calculated.
Cyanogenic glycoside determination
Cyanogenic glycoside was determined using alkaline picrate method. To the sample (5.0 g), 50 ml double distilled water was added in a conical flask and the mixture was allowed to stand overnight. The sample was filtered and 1 ml of the filtrate was taken into a corked test tube, 4 ml of alkaline picrate was added and incubated in a water bath for 5 min and colour changed from yellow to reddish brown after incubation for 5 min in a water bath to indicate presence of cyanide (Railes and Albrink 1981). The blank (containing 1 ml distilled water and 4 ml alkaline picrate solution) and sample readings were taken at absorbance of 490 nm.
Tannins determination
The samples (1.0 g) were dissolved in 10 ml double distilled water, vortex and left to stand for 30 min. at room temperature and centrifuged. The supernatant (2.5 ml) was measured into 50 ml volumetric flask. Similarly, 2.5 ml of standard tannic acid solution was measured into another 50 ml volumetric flask. To the sample and standard tannic solution, 1.0 ml of folin-dennis reagent was added and 2.5 ml of saturated Na2CO3 solution respectively and the mixtures were made to 50 ml in the flask with double distilled water and incubated for 90 min at room temperature. The absorbance of each sample was measured at 250 nm with the reagent blank at zero and the percentage tannin was calculated.
Trypsin inhibitor determination
Trypsin inhibition activity (TIA) was assayed in terms of the extent to which an extract of the defatted flour samples inhibited the action of bovine trypsin [EC 3.4.21.4] on the substrate benzoyl-DL-arginine-p-nitrianilide [BAPNA] hydrochloric. The blended flour samples (1 g each) were extracted continuously at ambient temperature for 3 h with 50 ml of 10 mmol/L NaOH using a mechanical shaker (Gallenkamp orbital shaker Surrey, UK). The pH of the resulting slurry was adjusted to 9.4–9.6 with 1 mol/L NaOH. After extraction, the suspension was shaken and diluted with double distilled water so that 1 ml of the extract produced trypsin inhibition of 40–60 % at 37 °C. The respective dilutions were noted. Consequently, TIA was calculated in terms of mg pure trypsin [Sigma type III, lot 20H0868] from the formula:
where: D is the dilution factor, A is the change in absorbance at 410 nm due to trypsin inhibition per ml of diluted sample extract, and S is the weight of the sample.
Animal experimental design
Sixty healthy Wistar albino rats (4 weeks of age) were divided into six groups of 10 animals each and were allowed to acclimatize to the new environment for 7 days, while being fed commercial feed and water ad libitum. After 7 days, the six groups of rats were fed the plantain-based functional foods (PSC-1, -2 and -3), 100 % plantain flour, Cerolina (a commercial food) and basal diets, with a diet per group and water ad libitum for 28 days. The rats were weighed every 4 days interval. Urine was collected from each cage in a small urine container, which contained about 1 ml concentrated sulfuric acid. Fecal samples were also collected daily, weighed, dried, and milled prior to laboratory analysis. The protein qualities of the food products were determined using the following calculations:
Nitrogen retention (NR; dietary nitrogen retained in the body):
Biological value (BV)
Food efficiency (FE):
Net protein utilization (NPU)
Protein efficiency ratio (PER)
True protein digestibility (TD)
Protein rating (PR):
where: Ni = nitrogen intake in proteins on the test diet, Nf = faecal nitrogen whilst on the test diet, Nef = feacal nitrogen excreted on nitrogen-free diet (metabolic nitrogen), Nu = urinary nitrogen whilst on the test diet, Neu = urinary nitrogen excreted on nitrogen-free diet (endogenous nitrogen).
Hematological determinations
Blood collection
At the end of the experimental period, all rats were starved for about 3 h and weighed. Each rat was anaesthetized with chloroform inside a dessicator before being sacrificed. Blood was collected into Bijou bottles containing a speck of dried tetra-acetic ethylenediamine acid powder, and hematological indices were determined.
Hematological analysis procedures
Packed cell volume (PCV) was estimated by centrifuging about 75 Fl of each blood sample in heparinized capillary tubes in a hematocrit micro-centrifuge for 5 min. Total red blood cell count (RBC) was determined using normal saline as the diluting fluid. Hemoglobin concentration (HBC) was estimated using the cyanomethemoglobin method and mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV) were calculated as follows: Mean corpuscular hemoglobin (MCH)
Mean corpuscular hemoglobin concentration (MCHC)
Mean corpuscular volume (MCV)
where Hb is the Hemoglobin
Determination of glycemic index and anti-diabetic potentials of formulated food samples in Wistar Albino rats
Experimental animals
Thirty-five Wistar albino rats (35) were weighed and divided into five groups containing seven rats each. The animals were allowed to acclimatize to their new environment for 7 days. After the adaptation period, the animals were reweighed and fasted for 12 h. The blood glucose of the animals were taken at zero time from the tail vein before feeding them with 2.0 g of the formulated food samples or glucose (control), which were consumed within 25 min. After the consumption, the serum glucose levels of the animals were measured using an automatic glucose analyzer (‘Accu-chek Active’ Diabetes monitoring kit; Roche Diagnostic, Indianapolis, USA).
Measurement of blood glucose response
Blood glucose curves were constructed from blood glucose values of animals at time 0, and 15, 30, 45, 60, 90 and 120 min intervals after consumption of the glucose (control) or formulated food samples of each group. The Incremental Area Under Curve (IAUC) was calculated for reference food (glucose) by the trapezoidal rule in every rats in each group separately as the sum of the surface of trapezoids between the blood glucose curve and horizontal baseline going parallel to x-axis from the beginning of blood glucose curve at time 0 to the point at time 120 min to reflect the total rise in blood glucose concentration after eating the reference food (glucose). The IAUC from the animals fed with the formulated food samples were similarly obtained. The glycemic Index (GI) for each diet was calculated by ratio of IAUC for each diet to the IAUC for glucose solution standard as reported by FAO/WHO (1991) using the following equation:
Calculation of glycemic load (GL)
Glycemic load (GL) for each food sample was determined by the method of Salmeron et al. (1997). Each individual glycemic load was calculated by taking the percentage of the food’s carbohydrate content in a typical serving food and multiplying it by its GI value. The following formula was used:
Net Carbohydrate = Total Carbohydrates in the food sample served.
Induction of diabetes mellitus
The baseline blood glucose levels of the animals were measured before being induced with alloxan drug. Fifty albino rats were induced by single intraperitoneal injection of freshly prepared solution of alloxan monohydrate (150 mg/kg. body weight) dissolved in physiological saline in overnight fasted Wistar Albino rats. The rats were allowed to drink 5 % glucose solution to avoid hypoglycaemic effects of the drug. The blood glucose levels in the animals were measured 72 h after alloxan administration through tail tipping using glucometer (‘Accu-chek Active’ Diabetes monitoring kit; Roche Diagnostic, Indianapolis, USA). The forty-two diabetic induced rats with serum glucose ≥250 mg/dl level were randomly divided into six groups containing seven rats each and four of the groups were fed with formulated food samples (PSC-1, PSC-2, PSC-3 and 100 % plantain). The remaining groups were treated with cerolina (a control sample) and metformin hydrochloride (an antidiabetic drug) respectively, and the rats in metformin group were fed with commercial animal feeds. The animals were fed with the diets for 28 days and blood glucose levels were measured in the morning at regular interval by drawing blood from each rat through tail tipping and the blood glucose level was measured using Glucometer kit (‘Accu-chek Active’ Diabetes monitoring kit; Roche Diagnostic, Indianapolis, USA)..
Sensory evaluation of dietary dough meal
Sensory evaluation was performed on the dietary dough meal samples using the descriptive 9-point Hedonic scale rating with 15 taste panelists that were familiar with the control sample (Cerolina). The rating was, 9 like extremely and 1, dislike extremely for each attribute evaluated. The assessments were conducted in a well lit room designed for sensory evaluation in the Department of Food Science and Technology. Dietary dough meal was prepared by stirring flour in boiling water 1:4 (v/v) of flour to water dispersion at 100 °C for 10 min. Panelists were from the University community and cut across age and sex.
Statistical analysis
The data were analysed using SPSS version 16.0. The mean and standard error of means (SEM) of the triplicate analyses were calculated. The analysis of variance (ANOVA) was performed to determine significant differences between the means, while the means were separated using the New Duncan Multiple Range Test (NDMRT) at p < 0.05.
Results
Proximate and mineral compositions
Proximate and mineral compositions of the plantain-based functional dough meal are presented in Table 1. Moisture content of the functional dough meals varied from 7.2 ± 0.01 in PSC-3 to 7.8 ± 0.01 g/100 g in PSC-1, and the values were comparable to that of Cerolina (7.45 ± 0.00 g/100 g). Crude protein content of the dough meals varied between 11.78 ± 0.07 g/100 g for PSC-2 and 16.49 ± 0.01 g/100 g for PCS-3, however, these values were significantly lower than the Cerolina. The results also showed that the protein contents of composite flours increased as the proportion of defatted soy cake flour added increased. The crude fiber content of functional dough meals varied from 1.29 ± 0.03 g/100 g for PCS-2 to 2.78 ± 0.06 g/100 g for PCS-3, and these values were significantly higher than 0.70 ± 0.02 g/100 g observed for Cerolina. The mineral compositions of functional dough meals are shown in Table 1. Potassium had the highest values varying from 18.80 ± 0.00 to 21.75 ± 0.00 mg/100 g, while zinc had the least values varying from 0.04 to 0.05 mg/100 g. The ranged value of calcium, magnesium and iron of the formulated diets were 1.40–2.40, 1.99–3.24 and 1.23–3.85 mg/100 g respectively, while that of Na/K molar ratio was 0.09–0.12. Comparatively, the values of Mg, Fe and Na/K molar ratio were significantly (p < 0.05) higher than that of Cerolina.
Table 1.
Nutrient (g/100 g) and mineral (mg/100 g) compositions of plantain-based functional dough meals supplemented with soybean cake and cassava fibre flours
| Parameters | PSC-1 | PSC-2 | PSC-3 | PLT | CER |
|---|---|---|---|---|---|
| Moisture | 7.80 ± 0.01a | 7.45 ± 0.03b | 7.2 ± 0.01c | 7.18 ± 0.05c | 7.45 ± 0.00b |
| Total ash | 3.78b ± 0.03b | 3.60 ± 0.01b | 5.36 ± 0.05a | 3.62 ± 0.11b | 1.85 ± 0.02c |
| Crude protein | 14.68 ± 0.03c | 11.78 ± 0.07d | 16.49 ± 0.01b | 8.25 ± 0.13e | 18.55 ± 0.02a |
| Crude fat | 4.26 ± 0.02d | 6.28 ± 0.03c | 8.85 ± 0.14a | 3.22 ± 0.21e | 7.41 ± 0.09b |
| Crude fibre | 2.29 ± 0.02b | 1.29 ± 0.03c | 2.78 ± 0.06a | 1.30 ± 0.07c | 0.70 ± 0.02d |
| Carbohydrate | 67.20 ± 0.04c | 69.48 ± 0.03b | 59.33 ± 0.04e | 76.41 ± 0.12a | 64.06 ± 0.12d |
| Energy (KJ/100 g) | 1528.18 ± 5.01c | 1593.81 ± 7.11b | 1599.84 ± 4.63b | 1535 ± 2.12c | 1658.94 ± 5.27a |
| Sodium | 1.89 ± 0.00b | 2.27 ± 0.00a | 2.29 ± 0.00a | 2.31 ± 0.07a | 0.77 ± 0.00c |
| Potassium | 21.20 ± 0.00b | 18.80 ± 0.00c | 21.75 ± 0.00b | 18.20 ± 0.05c | 63.15 ± 0.15a |
| Calcium | 2.30 ± 0.00a | 1.40 ± 0.00c | 2.40 ± 0.00a | 1.37 ± 0.04c | 2.20 ± 0.00b |
| Magnesium | 1.99 ± 0.00b | 3.24 ± 0.01a | 3.12 ± 0.00a | 3.11 ± 0.12a | 1.32 ± 0.00b |
| Iron | 3.85 ± 0.01a | 1.23 ± 0.03c | 1.84 ± 0.02b | 1.19 ± 0.03c | 0.15 ± 0.01d |
| Zinc | 0.04 ± 0.00c | 0.04 ± 0.00c | 0.05 ± 0.00b | 0.04 ± 0.00c | 0.07 ± 0.00a |
| Manganese | 0.04 ± 0.00b | 0.04 ± 0.00b | 0.06 ± 0.00a | 0.04 ± 0.00b | 0.06 ± 0.00a |
| Copper | 0.27 ± 0.00a | 0.04 ± 0.00b | 0.02 ± 0.00c | 0.04 ± 0.00b | 0.02 ± 0.00c |
| Na/K | 0.09b | 0.12a | 0.11a | 0.12a | 0.01c |
Values are Mean ± SEM. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain:75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %
Values with different alphabets across the row differ significantly at p < 0.05
CER Cerolina (control), PLT plantain (100 %)
Nutritional quality, amino acid and antinutrient compositions of plantain-based functional dough meal
In vivo protein digestibility and the amino acid profile of the plantain-based functional dough meals is presented in Table 2. The results showed that PSC-3 had the highest nitrogen retention (NR; 4.42), biological value (BV; 74.14 %), net protein utilization (NPU; 74.14 %) and true protein digestibility (TD; 92.89 %) when compared with PSC-1 and PSC-2. The non-essential amino acids had glutamic acid as the highest with values ranging from 17.07 mg/100 g protein in PSC-3 to 17.46 mg/100 g protein in PSC-1, while cysteine content was the lowest values, which ranges from 1.14 mg/100 g protein in PSC-1 to 1.63 mg/100 g protein in PSC-2. Leucine had the highest values (7.15–7.72 mg/100 g protein) in essential amino acids, while tryptophan was the lowest for PSC-1 and methionine for the PSC-2 and PSC-3. The essential amino acid scores showed that the first and second limiting amino acids of the formulations were methionine and valine respectively.
Table 2.
Protein quality and amino acid profile (mg/100 g protein) of plantain-based Functional dough meal supplemented with soybean cake and cassava fibre
| Parameters | PSC-1 | PSC-2 | PSC-3 | PLA | CER | *RDA |
|---|---|---|---|---|---|---|
| Feed intakes (g) | 145.05e | 154.56d | 232.56b | 157.85c | 261.01a | |
| Weight gained (g) | 38.08c | 26.88d | 47.8b | 19.61e | 54.97a | |
| Food efficiency | 0.26a | 0.17c | 0.21b | 0.12d | 0.21b | |
| Nitrogen retention | 1.95d | 1.56c | 4.42b | 0.55e | 7.25a | |
| Biological value (%) | 57.14c | 53.67d | 74.14b | 49.46e | 93.63a | |
| Net protein utilization (%) | 57.14c | 53.67d | 74.14b | 49.46e | 93.63a | |
| True protein digestibility (%) | 89.94c | 76.07d | 92.89b | 91.49b | 99.63a | |
| Protein Efficiency ratio | 0.26a | 0.17c | 0.21b | 0.12d | 0.21b | |
| Non-essential amino acids | ||||||
| Aspartic acid | 11.92b | 11.56b | 12.04a | 8.72c | – | |
| Tyrosine | 2.62a | 1.96c | 2.66a | 2.46b | – | |
| Serine | 5.65b | 5.69b | 5.82a | 5.23c | – | |
| Glutamic acid | 17.46b | 16.34c | 17.01b | 24.57a | – | |
| Proline | 7.20b | 7.57b | 7.61b | 10.65a | – | |
| Glycine | 3.51a | 2.39b | 3.36a | 1.22c | – | |
| Alanine | 4.95a | 3.66c | 4.82a | 4.10b | – | |
| Cysteine | 1.14c | 1.63a | 1.20c | 1.55b | – | |
| Arginine | 9.20a | 8.93ab | 8.68c | 6.93d | 2 | |
| Essential amino acids | ||||||
| Valine | 4.45a | 4.04ab | 3.80b | 4.23a | 3.5 | |
| Leucine | 7.23a | 7.72a | 7.15a | 7.70a | 6.6 | |
| Isoleucine | 3.25b | 3.03c | 4.93a | 3.21c | 2.8 | |
| Methionine | 1.23a | 1.25a | 1.14b | 1.24a | 2.2 | |
| Phenylalanine | 5.17c | 5.13c | 5.90a | 5.27b | 2.8 | |
| Lysine | 6.38a | 6.28a | 6.61a | 4.66b | 5.8 | |
| Histidine | 3.94a | 3.86a | 3.42b | 2.78c | 1.9 | |
| Tryptophan | 1.09b | 1.28a | 1.47a | 0.91c | 1.1 | |
| Threonine | 3.23b | 3.26b | 3.99a | 2.71c | 3.4 | |
| Predicted nutritional qualities | ||||||
| TEAA + His + Arg/TAA % | 49.30b | 48.85c | 51.72a | 43.22d | – | |
| TEAA/TAA % | 36.11b | 36.19b | 39.14a | 33.33c | – | |
| TNEAA/TAA % | 54.66a | 36.19c | 51.76b | 33.33d | – | |
| TSAA(Meth + Cys) | 2.37c | 2.45b | 2.77a | 2.79a | – | |
| ArEAA (Phe + Tyr) | 7.79b | 7.79b | 7.86a | 7.73b | – | |
| TEAA/TNEAA | 0.66b | 0.66b | 0.76a | 0.56c | – | |
| First Limiting amino acid | Methionine | Methionine | Methionine | Methionine | – | |
| Second Limiting amino acid | Valine | Valine | Valine | Valine | – | |
Values are Mean ± SEM. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain:75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %. * Recommended daily allowance (WHO/FAO 1991)
Values with different alphabets across the row differ significantly at p < 0.05
CER Cerolina (control), PLT plantain (100 %)
The haematological properties of plantain-based functional dough meal are presented in Table 3. The packed cell volume (PCV) of the rats fed with the dough meal ranged from 27 to 35 %, red blood cells ranged from 3.0 × 106 to 3.95 × 106 mm3, white blood cells (WBC) ranged from 5.0 × 103 to 8.6 × 103 mm3, while haemoglobin concentration (Hb) ranged from 9.1 to 11.7 (Table 3). The range values of mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) of the rats fed with the experimental diets were 27.03–33.33 fL, 2.96–3.03 pcg and 33.43–33.70 g/dL respectively.
Table 3.
Haematological indices of Albino rats fed with plantain-based functional dough meal supplemented with soybean cake and cassava fibre
| Parameters | PSC-1 | PSC-2 | PSC-3 | PLT | CER |
|---|---|---|---|---|---|
| Erythrocyte sedimentation rate (mm/h) | 0.5a | 0.5a | 0.2b | 0.2b | 0.5a |
| Packed cell volume (%) | 35.0a | 27.0b | 33.0a | 29.0b | 34.0a |
| Red blood cells (×106mm3) | 3.95a | 3.0b | 3.7a | 3.2b | 3.8a |
| White blood cells (×103mm3) | 5.8c | 8.6a | 5.0c | 6.2b | 6.1b |
| Haemoglobin (g/dL) | 11.7a | 9.1b | 11.1a | 8.7c | 11.3a |
| Lymphocytes (%) | 36b | 29c | 35b | 40a | 40a |
| Neutrophils (%) | 64b | 69a | 62cd | 58d | 60cd |
| Monocytes (%) | 2a | 2a | 2a | 2a | 2a |
| Eosinophils (%) | 2a | 2a | 1a | 2a | 2a |
| Basophils (%) | 1a | 1a | 2a | 1a | 1a |
| Mean corpuscular volume (MCV; fL) | 25.32d | 33.33a | 27.03c | 31.25b | 26.32d |
| Mean corpuscular haemoglobin (MCH; pcg) | 2.96b | 3.03a | 3.00a | 2.72c | 2.97b |
| MCH concentration (MCHC; g/dL) | 33.43c | 33.70a | 33.64b | 30.00e | 33.24d |
Values are Mean ± SEM. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain:75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %
Values with different alphabets across the row differ significantly at p < 0.05
CER Cerolina (control), PLT plantain (100 %)
Anti-nutritional compositions of the dough meals are shown in Table 4. The concentration varied as follows: oxalate (0.45 ± 0.00–0.68 ± 0.00 mg/g), tannins (0.03 ± 0.00–0.07 ± 0.00 mg/g), phytate (8.93 ± 0.01–12.18 ± 0.81 mg/g), trypsin inhibitor (13.26 ± 0.22–21.15 ± 0.17 %) and hydrogen cyanide (0.24 ± 0.00–0.30 ± 0.00 mg/100 g). All the values are within the ranges obtained for the control sample.
Table 4.
Antinutrient composition of plantain-based functional dough meal supplemented with soybean cake and cassava fibre
| Parameters | Phytate (mg/g) | Oxalate (mg/g) | Tannin (mg/g) | Trypsin (%) | Cyanide (mg/100 g) |
|---|---|---|---|---|---|
| PSC-1 | 12.18 ± 0.81a | 0.45 ± 0.00c | 0.03 ± 0.00d | 13.26 ± 0.22d | 0.27 ± 0.00ab |
| PSC-2 | 8.93 ± 0.01c | 0.68 ± 0.05a | 0.05 ± 0.00c | 21.15 ± 0.17a | 0.30 ± 0.00a |
| PSC-3 | 10.15 ± 0.41b | 0.54 ± 0.00b | 0.07 ± 0.00b | 19.45 ± 0.00b | 0.24 ± 0.00b |
| PLT | 7.28 ± 0.04d | 0.65 ± 0.03a | 0.05 ± 0.00c | 12.22 ± 0.21e | 0.13 ± 0.02c |
| CER | 3.25 ± 0.81e | 0.68 ± 0.05a | 0.11 ± 0.00a | 15.77 ± 0.00c | 0.30 ± 0.00a |
Values are Mean ± SEM. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain:75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %
Values with different alphabets across the column differ significantly at p < 0.05
CER Cerolina (control), PLT plantain (100 %)
Glycaemic index, glycaemic load and anti-diabetic potential of plantain-based dough meal
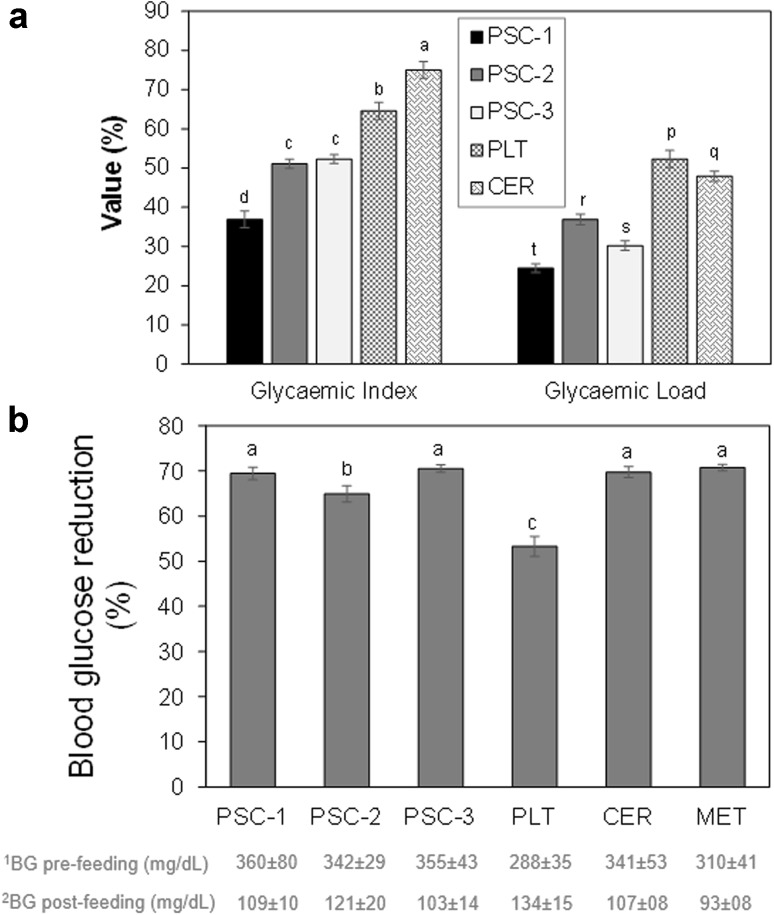
The glycaemic index (GI) and glycaemic load (GL) of the meal samples are presented in Fig. 1. The result showed that PSC-1 (36.9 %) sample had the lowest glycaemic index while PSC-2 (55.1 %) had the highest of the three. Similarly, PSC-1 had the lowest glycaemic load (GL; 24.5 %) and other samples had GL that correlate positively with the GI (Fig. 1a).
Fig. 1.
Glycaemic index and Glycaemic load (a) of dough meal supplemented with soybean cake and cassava fibre, and Percentage of blood glucose reduction (b) in Wistar rats fed with the experimental diets, control and metformin. Values are Mean ± SEM. Bars with different alphabet show that they are significantly different (p < 0.05). GI classification: Low-GI = <55 %; Medium-GI = 56–69 %; High-GI = >70. GL classification: Low-GL = <10; Medium-GL = 11–19; High-GL = >20. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain: 75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %. CER Cerolina (Control), PLT plantain (100 %), MET metformin (a synthetic antidiabetic drug). 1Blood glucose level in diabetic rats after being induced with aloxan. 2Blood glucose level in rat after feeding them with formulated diets, metformin or cerolina
The anti-diabetic potential of plantain-based functional dough meals is presented in Fig. 1b. The result showed that there was significant difference between the percentages of blood glucose reduction in rats fed with plantain-based functional dough meals when compared with the rats fed with 100 % plantain flour alone. However, there was no significant difference (p < 0.05) between the rats fed with functional dough meals, particularly PSC-1 and PSC-3, and those rats fed with Cerolina and those administered with Metformin (a synthetic antidiabetic drug) groups.
Sensory attributes of plantain-based functional dough meal
The sensory attributes of the functional dough meals are presented in Table 5. The PSC-1 sample was significantly rated higher in terms of appearance, mold-ability, mouthfeel, aroma, texture and overall acceptability when compared with other dough meals and control samples. There was no significant difference between the PSC-1 and PSC-2 samples, but there was significant difference between these samples and that of PSC-3 sample. This observation could be due to the level of soy bean cake substituted into the plantain flour, which was 25 % in PSC-3 compared with 19 % and 15 % in PSC-1 and PSC-2 respectively.
Table 5.
Sensory attributes of plantain-based functional dough meal supplemented with soybean cake and cassava fibre
| Parameters | Appearance | Moldability | Mouth feel | Aroma | Texture | OA | Mean |
|---|---|---|---|---|---|---|---|
| PSC-1 | 7.13 ± 0.92a | 7.27 ± 1.16a | 7.60 ± 1.24a | 6.02 ± 1.89a | 7.07 ± 1.28a | 7.33 ± 0.98a | 7.07 ± 1.02a |
| PSC-2 | 6.47 ± 1.19a | 6.93 ± 1.22a | 7.61 ± 1.40a | 6.01 ± 2.24a | 6.80 ± 0.86a | 7.20 ± 0.77a | 6.83 ± 1.03a |
| PSC-3 | 6.33 ± 1.29a | 4.53 ± 1.92b | 5.87 ± 1.41b | 6.27 ± 2.09a | 5.82 ± 2.11a | 6.01 ± 1.51b | 5.80 ± 1.11c |
| PLT | 6.40 ± 1.12a | 6.71 ± 1.21a | 7.52 ± 1.24a | 6.35 ± 1.17a | 6.55 ± 0.77a | 7.11 ± 0.69a | 6.77 ± 1.01a |
| CER | 7.13 ± 2.03a | 5.27 ± 1.75b | 7.0 ± 1.36a | 6.67 ± 1.11a | 6.21 ± 1.93a | 6.73 ± 2.12ab | 6.50 ± 1.02b |
Values are Mean ± SEM. Where: PSC-1 = Soycake: 19.01 %; Cassava fibre: 5.0 %; Unripe plantain: 75.99 %. PSC-2 = Soycake: 15.0 %; Cassava fibre: 8.0 %; Unripe plantain: 77.0 %. PSC-3 = Soycake: 25.0 %; Cassava fibre: 8.0 %; Unripe plantain: 67.0 %
Values with different alphabets across the column differ significantly at p < 0.05
CER Cerolina (control), PLT plantain (100 %), OA overall acceptability
Discussion
The dough meals produced were very low in moisture content which is a determinant of its storage stability and the low moisture content of the dough meals is an indication that the plantain-based foods will keep long during storage. Protein supplementation (soy) for Type 2 diabetic patient has a therapeutic effect for both glycemic control and for cardiovascular risk (Jayagopal et al. 2002). The relatively higher protein content will make the current dough meal an excellent meal for diabetic patient. The incorporation of cassava fiber into the formulations resulted in increase in the crude fibre content of the dough meal. Studies have shown that consumption of fibers have positive benefits on health by increasing the volume of feacal bulk and thereby decreasing the time of intestinal transit, reducing cholesterol, glycemic levels and trapping mutagenic and carcinogenic agents (Beecher 1999). Improvements in diabetic control, reduction in blood glucose, insulin and sulfonylurea medications have been reported for fiber intakes in diabetic patients (Devinder et al. 2012).
The formulated diets were significantly higher in sodium, magnesium and iron than the control sample, but lower in potassium, calcium and zinc. The variation between the mineral compositions of formulated diets and Cerolina could be attributed to the differences in raw food materials, geographical sources of the food materials and the processing techniques. Findings have shown that low dietary intake of sodium and high dietary intake of potassium, calcium, and magnesium reduced the risk of cardiovascular disease, by lowering blood pressure and the risk of diabetes (Chatterjee et al. 2012). However, the Na/K ratio of the formulated diets are low enough which enhanced the dietary potential of the formulated diets.
Analysis of the amino acid composition showed that the dough meals were higher in aspartic acid, glutamic acid, glycine and arginine when compared with control sample. Nutritionally, the essential amino acids in the dough meals were higher in valine, leucine, isoleucine phenylalanine, lysine and histidine compared with FAO/WHO (1991) recommended daily allowances (RDA). The percentage ratios of TEAA to TAA in the samples ranges from 48.85 to 51.72 %, which were well above the 39 % considered to be adequate for ideal protein food for infants, 26 % for children and 11 % for adults (FAO/WHO 1991). Also, a food product with biological values of >70 is rated to be of good quality. With the values obtained in the current study, it implies that the plantain-based dough meals, particularly PSC-3 with 25 % soybean cake and 8 % cassava fibre, can adequately provide the daily essential amino acids for the consumers.
Hematological analysis of rat fed with the dough meals showed PCV, Hb, RBC, WBC, MCV, MCH and MCHC to be comparatively lower than values obtained for similar parameters in rats fed with similar product made of plantain-cowpea (Olapade et al. 2015). Finding showed that PCV, RBC and Hbc concentration decreased in anaemic condition while WBC influenced the immune response as high WBC was reported to the synonymous with microbial infection (Aletor and Egberongbe 1992).
Antinutritional factors are the major barriers to the use of legumes in the formulation of complementary diet as phytic acid chelates metal ions, especially zinc, iron, and calcium to form insoluble complexes in the gastrointestinal tract, and thereby reducing bioavailability of these vital minerals and protein for the normal growth and development (Soetan and Oyewole 2009). However, the anti-nutrients in the dough meals were found to be lower than the lethal doses of 2–5 g/kg for oxalate, 50–60 mg/kg for phytate, 30 mg/kg for tannin, 10 mg/kg for cyanogenic glycoside and 2.50 g/kg for trypsin inhibitor (Nwosu 2011).
Glycaemic Index (GI) is a measure of the effects of carbohydrates on blood glucose levels and a tool for the dietary management of Type II diabetes (Brand-Miller et al. 2003; Sun et al. 2010). Low GI foods reduce postprandial blood glucose levels and this knowledge can be used in recommending and planning meals for diabetic patients (Hermansen et al. 2006; Agama-Acevedo et al. 2012). The soybean cake and cassava fibre in the plantain-based dough meal are pivotal to the low GI and GL observed. Low GI and GL foods produce a low plasma glucose concentration as a result of slower rates of gastric emptying and digestion of carbohydrate in the intestinal lumen and subsequently, a slower rate of absorption of glucose into the portal and systemic circulation hence, reduce the risk of coronary heart disease and type 2 diabetes (Liu et al. 2000; Salmeron et al. 1997), hence, information on diets GI may be useful in planning carbohydrate-based foods for individuals with diabetes (Hermansen et al. 2006). Study has shown that consumption of unripe plantain or bananas confers beneficial effects for human health, due to its high resistant starch (RS) content (Sajilata et al. 2006; Hernández-Nava et al. 2009; Ovando-Martinez et al. 2009). Eleazu et al. (2010) reported that unripe plantain pulp could be used to manage type 2 diabetes mellitus, and that it is usually eaten in boiled, fried, roasted or dough meal ‘amala’ forms.
Oral hypoglycaemic drugs such as the sulphonylureas and biguanides commonly used in the management of type 2 diabetes have side effects that include haematological, cutanecous, gastrointestinal reactions and disturbances in both liver and kidney. Also, the cost of the drugs coupled with an exponential increase in the prevalence of diabetes mellitus are impetus for formulation of functional foods as possible alternative therapies for the management of diabetes mellitus (Shodehinde et al. 2014).
Conclusion
Plantain-based dough meals developed with the incorporation of soycake and cassava fibre were found to be comparable to commonly consumed cerolina (a commercial food products) in terms of nutritional profile and glycaemic index. The hypoglycaemic properties of the dough meals showed that they compare favorably with metformin (a synthetic hypoglycaemic drug) in blood glycaemic control activities. Hence, the formulated plantain-based functional dough meals have potentials suitable for the prevention and management of diabetes mellitus.
Compliance with ethical standards
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Akindele Fatoyinbo, FAX: 2348034795624.
Tayo Nathaniel Fagbemi, Phone: +2348036669711, Email: tnfagbemi@futa.edu.ng.
References
- Abiose SH, Ikujenlola AV, Abioderin FI. Nutritional quality assessment of complementary foods produced from fermented and malted quality protein maize fortified with soybean flour. Pol J Food Nutr Sci. 2015;65:49–56. [Google Scholar]
- Agama-Acevedo E, Islas-Hernández JJ, Pacheco-Vargas G, Osorio-Díaz P, Bello-Pérez LA. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour. LWT Food Sci Technol. 2012;46:177–182. doi: 10.1016/j.lwt.2011.10.010. [DOI] [Google Scholar]
- Agwu AE, Anyaeche CL. Adoption of improved cassava varieties in six rural communities in Anambra state, Nigeria. African J Biotechnol. 2007;6:89–98. [Google Scholar]
- Aletor VA, Egberongbe O. Feeding differently processed soya bean. Part 2: an assessment of haematological responses in the chicken. Food/Nahrung. 1992;36:364–369. doi: 10.1002/food.19920360406. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Analytical Chemists) Official methods of analysis. In: Horowitz W, editor. Official methods of analysis. Gaithersburg: AOAC; 2005. [Google Scholar]
- Beecher GR. Phytonutrients role in metabolism: effects on resistance to degenerative processes. Nutr Rev. 1999;57:3–6. doi: 10.1111/j.1753-4887.1999.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in management of diabetes: a meta-analysis randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- Buzzigoli G, Lanzone L, Ciorciaro D, Frascerra S, Cerri M, Scandroglio A, Coldani R, Ferrannini E. Characterization of a reversed-phase high performance liquid chromatographic system for determination of blood amino acids. J Chromatogr A. 1990;507:85–93. doi: 10.1016/S0021-9673(01)84184-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee R, Colangelo LA, Yeh HC, Anderson CA, Daviglus ML, Liu K, Brancati FL. Potassium intake and risk of incident type 2 diabetes mellitus: the coronary artery risk development in young adults (CARDIA) study. Diabetologia. 2012;55:1295–1303. doi: 10.1007/s00125-012-2487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RA, Underwood AL. Quantitive analysis. 5. New Delhi: Prentice-Hall Publication; 1986. p. 701. [Google Scholar]
- Devinder D, Mona M, Hradesh R, Patil RT. Dietary fibre in foods: a review. J Food Sci Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleazu CO, Okafor PN, Ikpeama A. Total antioxidant capacity, nutritional composition and inhibitory activity of unripe plantain (Musa paradisiaca) on oxidative stress in alloxan induced diabetic rabbits. Pak J Nutr. 2010;9:1052–1057. doi: 10.3923/pjn.2010.1052.1057. [DOI] [Google Scholar]
- FAO/WHO . Protein quality evaluation. Food and Agricultural organization of the United Nations: Roe, Italy; 1991. pp. 1–66. [Google Scholar]
- Franz MJ, Horton ES, Bantle JP, Beebe CA, Brunzell JD, Coulston AM, Henry RR, Hoogwerf BJ, Stacpoole PW. Nutrition principles for the management off diabetes and related complications. Diabetes Care. 1994;17:490–518. doi: 10.2337/diacare.17.5.490. [DOI] [PubMed] [Google Scholar]
- Gandhi AP. Quality of soybean and its food products. Intern Food Res J. 2009;16:11–19. [Google Scholar]
- Glew RS, Vanderjagt DJ, Chauang LT, Huang YS, Millson M, Glew RH. Nutrient content of four edible plants from plants from West Africa. Plant Foods Human Nutr. 2005;60:187–193. doi: 10.1007/s11130-005-8616-0. [DOI] [PubMed] [Google Scholar]
- Hermansen MF, Nina MBE, Lene SM, Lotte H, Kjeld H. Can the Glycemic Index (GI) be used as a tool in the prevention and management of type 2 diabetes? Rev Diabet Stud. 2006;3:61–71. doi: 10.1900/RDS.2006.3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Nava RG, Berrios J, Pan J, Osorio-Díaz P, Bello-Pérez LA. Development and characterization of spaghetti with high resistant starch content supplemented with banana starch. Food Sci Technol Intern. 2009;15:73–78. doi: 10.1177/1082013208102379. [DOI] [Google Scholar]
- Idowu MA, Oni A, Amusa BM. Bread and Biscuit making potential of some Nigerian cocoyam cultivars. Nigeria Food J. 1996;14:1–12. [Google Scholar]
- Jayagopal V, Albertazzi P, Kilpatrick MS, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with Type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Kapoor SK, Anand K. Nutritional transition: a public health challenge in developing countries. J Epidemiol Community Health. 2002;56:804–805. doi: 10.1136/jech.56.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- Lolas GM, Markakis P. Phytic acid and other phosphorus compounds of bean (Phaseolus vulgaris) J Agric Food Chem. 1975;23:13–15. doi: 10.1021/jf60197a016. [DOI] [Google Scholar]
- Nwosu JN. The effects of processing on the anti-nutritional properties of Oze Bosqueia angolensis seed. J Am Sci. 2011;7:1–6. [Google Scholar]
- Odenigbo AM, Asumugha VU, Ubbor S, Nwauzor C, Otuonye AC, Offia-Olua BI, Princewill-Ogbunna IL, Nzeagwu OC, Henry-Uneze HN, Anyika JU, Ukaegbu P, Umeh AS, Anozie GO. Proximate composition and consumption pattern of plantain and cooking-banana. British J Appl Sci Technol. 2013;3:1035–1043. doi: 10.9734/BJAST/2013/4943. [DOI] [Google Scholar]
- Olapade AA, Babalola KA, Aworh OC. Evaluation of plantain and cowpea blends for complementary foods. Agric Food. 2015;3:274–288. [Google Scholar]
- Osundahunsi OF. Functional properties of extruded soybean with plantain flour blends. J Food Agric Environ. 2006;4:57–60. [Google Scholar]
- Ovando-Martinez M, Sáyago-Ayerdi SG, Agama-Acevedo E, Goñi I, Bello-Pérez LA. Unripe banana flour as an ingredient to increase the indigestible carbohydrates of pasta. Food Chem. 2009;113:121–126. doi: 10.1016/j.foodchem.2008.07.035. [DOI] [Google Scholar]
- Popkin BM. The shift in stages of the nutrition transition in the developing world differs from past experiences. Public Health Nutr. 2002;5:205–214. doi: 10.1079/PHN2001295. [DOI] [PubMed] [Google Scholar]
- Railes R, Albrink MJ. Effect of chromium chloride supplementation on glucose tolerance and serum lipids including high-density lipoprotein of adult men. Am J Clin Nutr. 1981;34:2670–2678. doi: 10.1093/ajcn/34.12.2670. [DOI] [PubMed] [Google Scholar]
- Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch—A review. Comp Rev Food Sci Food Safety. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load and risk of non-insulin-dependent diabetes mellitus in women. J Am Med Assoc. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- Shaw J, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shodehinde SA, Oboh G, Nwanna EE. Capacity of boiled green plantain (Musa paradisiaca) on the enzymes linked with diabetes and hypertension in comparison with synthetic drugs in Vitro. Intern J Curr Microbiol App Sci. 2014;3:964–976. [Google Scholar]
- Soetan KO, Oyewole OE. The need for adequate processing to reduce the antinutritional factors in plants used as human foods and animal feeds: a review. African J Food Sci. 2009;3:223–232. [Google Scholar]
- Sun Q, Spiegelman D, Van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–969. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]