Abstract
Millions of tons of fruit byproducts are discarded globally every day by food processing industries, which represents a considerable loss in terms of nutrients. The goal of this work was to evaluate the physico-chemical, technological, antioxidant properties and characterization of carotenoids for papaya, pineapple, olive byproducts and anthocyanins for blueberry byproducts. The results indicated that these byproducts are good sources of total dietary fiber, especially olive byproducts (53.68%). The powder from papaya byproducts showed the highest values for the technological characteristics of water holding capacity (8.93 g water/g powder) and solubility (59.91%). All of the powders exhibited a good ability to reduce Folin Ciocalteu reagent and a high DPPH radical scavenging capacity, especially the powder from blueberry byproducts, which also exhibited a high level of anthocyanins (2063.4 mg/100 g). The carotenoid content was highest in the papaya powder sample, followed by the pineapple and olive powders. The results of this study indicate the high potential application of these powder byproducts as functional ingredients in food products because they can be considered a good source of antioxidant compounds.
Keywords: Byproducts, Fiber, Powder, Antioxidants
Introduction
A large quantity of byproducts is generated daily by the food processing industry, primarily due to the production of juices, jellies, candies and fresh-cut fruits. It is estimated that whole processed fruits can generate byproducts comprising approximately 30–90% of these fruits, including peels, seeds and pulp. In the fresh-cut fruit industry, the byproducts are mainly peels and seeds of different shapes and sizes (Ayala-Zavala et al. 2011).
These byproducts can present fibers and bioactive components, such as phenolic compounds, carotenoids and anthocyanins. However, they are typically used to produce animal feed or they are discarded, which causes a significant environmental problem (O’shea et al. 2012; Tarazona-Díaz and Aguayo 2013). Considering their functional, technological and nutritional properties, these byproducts can be applied as food ingredients, providing outstanding health benefits.
Byproducts from the fruit processing industry contains high total dietary fiber content. Dietary fiber has been underlined as a healthy component for humans because of its capacity to protect against coronary disease and to reduce cholesterol, diabetes and constipation (Figuerola et al. 2005). Furthermore, the byproducts present other properties that allow its use as food additives, such as antimicrobials, antioxidants, flavorings, pigments, and thickening agents (Ayala-Zavala et al. 2011).
Byproducts from different fruits and vegetables have been evaluated, such as those from apple, potato, cucumber, melon and watermelon (Tarazona-Díaz and Aguayo 2013), mango (Ajila et al. 2008), and pineapple, guava and passion fruit (Martínez et al. 2012). However, the studies did not characterize completely the functional and antioxidant profiles of these byproducts, specially related to powder obtained from it. Most of the researches generally reported only one characterization profile or used part of the residue (Saikia and Mahanta 2015; Morais Ribeiro da Silva et al. 2014; O’shea et al. 2012).
The application of these byproducts in food is a promising field for the food industry. The addition or replacement of wheat powder by byproducts powder with high content of fiber and bioactive compounds in products consumed every day by the general population, as bakery products (Bhol et al. 2015; Singh et al. 2015), is essential and viable (Lima et al. 2014). This would be a way to supply the intake of these compounds so important to the health of people (Elleuch et al. 2011).
Therefore, this study aimed to determine the chemical, technological and in vitro antioxidant properties of the powder obtained from byproducts of processing fruits, including pineapple, papaya, blueberry and olive.
Materials and methods
Byproducts
Fresh pineapple byproducts were provided by the “Puro Suco” Company located in Porto Alegre (RS/Brazil). Olive byproducts were provided by the “Olivas do Sul” Company, Cachoeira do Sul (RS/Brazil). Papaya fruit (Formosa) was purchased in Central de Abastecimento (CEASA), Porto Alegre (RS/Brazil), and organic blueberries (Vaccinium spp.), fruit of the cultivar Delite (Rabbiteye group), were obtained from the “Fazenda Viva o Verde”, Camaquã (RS/Brazil). The fruits were procured at the same time and were processed in the Laboratory of Bioactive Compounds at UFRGS. The blueberry residue (bagasse) was obtained using a domestic centrifugal-type extractor (Walita-Philips®) in which the juice was separated from the residue (pomace). The papaya peels were obtained manually. The residues were subsequently stored at −18 °C until analysis. Pineapple and papaya byproducts were composed only of peels, and the olive and blueberry byproducts were composed of peels, pulp and seeds.
Powder production
The powder of the byproducts was prepared according to Crizel et al. (2013). The powder was produced by drying the residue in an oven with forced air circulation (DeLeo, Model B5AFD,Brazil) at 55 °C for 24 h and subsequently grinding it in a mill (Bertel Brand, Model MCF55, Brazil). The milled powder was separated using sieves for particle size analysis (Bertel, Brazil); Particles smaller than 125 mm were separated. The powder was packed in a vacuum sealer (Fastvac, model F200, Brazil) and maintained in the dark at room temperature (25 °C).
Analysis
Proximate composition
The powders were analyzed in triplicate according to the AOAC (1990). The carbohydrate content was assessed by the difference. The results were presented in g per 100 g of dry matter (DM).
Powder dietary fiber composition
The total dietary fiber, soluble fiber and insoluble fiber measured by enzyme-gravimetric method according to AOAC (1990) (method 991.43).
Functional properties
The water holding capacity (WHC) was determined using a method similar to that of the OHC, substituting the sunflower oil with distilled water. The result was expressed as g of water per g of dry powder sample.
The oil holding capacity (OHC) analysis was performed according to Fernández-López et al. (2009) with minor adjustments. Thirty milliliters of sunflower oil was added to 1 g of fiber sample, and the suspension was homogenized in a vortex (Quimis, Model Q920-A2, Brazil) for 1 min, followed by storage for 24 h at room temperature. After centrifugation (3000g for 20 min, Sigma, model 4K15, England), the supernatant was withdrawn, and the residue was weighed. The oil retention capacity was expressed in g of oil per g of dry powder sample.
The solubility analysis of the powders was performed according to Cano-Chauca et al. (2005).
Determination of bioactive compounds
Preparation of the extracts
For the phenolic compound and DPPH analyses, 1 g of powder sample was used, and the extraction was carried out with 50% methanol (20 mL × 1) and 70% acetone (20 mL × 1) in an Ultra Turrax® homogenizer (IKA Ultra Turrax digital, T25, Germany) for 1 min. Then, the sample was left for 60 min at room temperature (25 °C). The extract was centrifuged (25,000g for 15 min, Hitachi, model CR216III, Japan), and the supernatant was stored in an amber flask.
Total phenolic content
The total phenolic content of different powders obtained from fruit byproducts were determined using the Folin-Ciocalteu spectrophotometric method. According to Singleton and Rossi (1965), gallic acid was used as a standard, and the absorbance was measured at 725 nm. For quantification, an analytical calibration curve was constructed (measurements in triplicate), and the results are expressed as mg of gallic acid equivalents (GAE) per g of dry sample.
Antioxidant activity-DPPH method
The method is based on the capture of the 2.2-diphenyl-1-picrylhydrazyl (DPPH·) radical by antioxidant compounds present in extracts, producing a decrease in the absorbance at 515 nm (Rodrigues et al. 2014). From the extract previously described (“Preparation of the extracts” section), solutions with different dilution factors were prepared. For the antioxidant activity analysis, 100 μL of each diluted extract was combined with 3900 μL of DPPH solution, and after 40 min of reaction time, the readings were taken with a spectrophotometer (set to 515 nm) using methanol as a control (Brand-Williams et al. 1995). The extract samples were analyzed in triplicate, and the results are expressed as the concentration of antioxidant necessary to reduce the initial amount of free radicals by 50% (EC50). The IC50 value is the final concentration in mg/mL of the dry extract required to decrease or inhibit 50% of the initial DPPH concentration, and it was determined by linear regression. An test to detect interference was performed only on the extract without the addition of DPPH.
Carotenoid extraction, identification and quantification
The carotenoid extracts of papaya, olive and pineapple byproduct powders were prepared according to Mercadante et al. (1998). The pigments were extracted with chilled acetone in an Ultra Turrax® homogenizer (IKA Ultra Turrax digital, T25, Germany) until the sample was completely discolored. Then, the extracts were saponified overnight with 10% KOH in a methanol solution in the dark and at room temperature. Then, the extracts were washed to eliminate the alkali content and were concentrated using a rotary evaporator (Fisatom, model 801/802, Brazil). The concentrated extract was placed to an amber flask, subjected to a stream of nitrogen until it was completely dried and stored in freezer at −18 °C until the moment of analysis in high performance liquid chromatography (HPLC).
HPLC analysis was performed on an Agilent 1100 Series HPLC system equipped with a quaternary solvent pumping system (G1311A–DE14917573 Agilent 1100 Series) and a UV/Vis detector (G1314B–DE71358944 Agilent 1100 Series). A 250 mm × 4.6 mm i.d., 3 µm, C30 reversed phase polymeric column was applied (YMC, Japan). The wavelength was set in 450 nm. The mobile phase was water:methanol:tert-methyl butyl ether (MTBE) (J.T. Baker and Mallinckrodt, EUA) with the gradient starting at 5:90:5, reaching 0:95:5 in 12 min, reaching 0:89:11 in 25 min, reaching 0:75:25 in 40 min, finally reaching 0:50:50 at 50 min applying a flow rate of 1 mL/min at 33 °C (Zanatta and Mercadante 2007). The injection volume of the extract was 5 µL and the total run time was 50 min.
Standard curves of cryptoxanthin (4–100 mg/L), zeaxanthin (1–40 mg/L), lutein (1–65 mg/L), β-carotene (5–50 mg/L) and α-carotene (2–25 mg/L) were used to quantify the carotenoids. The standard of carotenoids cryptoxanthin (purity > 97%), zeaxanthin (purity > 95%), β-carotene (purity > 93%) and α-carotene (purity > 95%) and were acquired from Sigma Chemical (USA). Lutein (purity > 95%) was obtained from Indofine Chemical Company Inc. (Hillsborough, NJ, USA). The results are expressed in milligrams per 100 g of dry sample.
Anthocyanin extraction, identification and quantification
The anthocyanins were exhaustively extracted from the powder of blueberry byproducts. Approximately 1 g of the powder was mixed with 1% HCl in methanol in an Ultra Turrax® homogenizer. The injection into the HPLC to quantify the anthocyanins was carried out on the same day as the extraction because the extracts were not concentrated in the rotary evaporator. The anthocyanins were identified in comparison with the appropriate standards. HPLC analysis was performed using the same system as that used for the carotenoid analysis. However, to separate the anthocyanins, a C18 CLC-ODS reversed phase column (250 mm × 4.6 mm i.d., 5 µm, Shimadzu, Kyoto, Japan) was used, which was different from that used for the carotenoid analysis. A linear gradient elution of a mobile phase consisting of 4% aqueous phosphoric acid:acetonitrile from 85:15 (v/v) to 20:80 (v/v) after 12 min was used according to the conditions established experimentally by Zanatta et al. (2005). The flow rate was 1.0 mL/min, and the injection volume was 5 μL. The temperature of the column was maintained at 29 °C, and Chromatograms were acquired in 520 nm. The extraction and injection into the chromatograph of the anthocyanin extracts were performed in triplicate, and the compounds were identified comparing retention times (tR) obtained for samples with the standards purchased from Sigma-Aldrich® (St. Louis, MO, USA).
Standard curves were determined with glycosylated anthocyanins, such as cyanidin-3-glucoside (Sigma-Aldrich, 95% purity), delphinidin-3-β-d-glucoside (Santa Cruz Biotechnology, 95% purity), pelargonidin-3-glucoside (Sigma-Aldrich, 97% purity) and malvidin-3-glucoside (Sigma-Aldrich, 90% purity), as well as the aglycones of the anthocyanins, such as aglycone delphinidin (Sigma-Aldrich, 95% purity), aglycone cyanidin and aglycone malvidin.
Statistical analysis
The results were evaluated by analysis of variance (ANOVA) and Tukey’s test at a significance level of 0.05 using Statistica 11.0 software (STATSOFT Inc.).
Results and discussion
Proximate composition
In Table 1, the approximate composition of the various types of powders is presented. The proximal composition of the byproduct powders showed significant difference (p < 0.05) among the different sources related to the moisture, lipids, ash, proteins and carbohydrates.
Table 1.
Chemical compositions of the powders from pineapple, papaya, blueberry and olive byproducts
| Byproduct powder | Moisture (g/100 g DM)* | Proteins (g/100 g DM)* | Lipids (g/100 g DM)* | Ash (g/100 g DM)* | Carbohydrates (g/100 g DM)*,a |
|---|---|---|---|---|---|
| Pineapple | 13.56 ± 0.43b | 6.72 ± 0.34b | 1.02 ± 0.01d | 4.58 ± 0.04b | 87.68 ± 0.39a |
| Papaya | 15.85 ± 0.57a | 17.72 ± 0.81a | 2.48 ± 0.40c | 9.09 ± 1.76a | 70.71 ± 1.73c |
| Blueberry | 7.91 ± 0.59c | 7.55 ± 0.33b | 4.13 ± 0.19b | 4.14 ± 0.17b | 84.18 ± 0.45b |
| Olive | 1.41 ± 0.08d | 5.27 ± 0.53c | 23.08 ± 0.42a | 3.87 ± 0.15b | 67.78 ± 0.58d |
DM dry matter
* Results are the means of three determinations ± standard deviation. Different letters in the same line are significantly different as determined by Tukey’s test (p ≤ 0.05)
aDetermined by the difference
The papaya byproduct powder presented the highest moisture content. This may be due to differences in the initial moisture contents of each byproduct because the drying time and temperature were the same for all of the powders. The powder from olive byproducts showed the lowest moisture content, which can be explained by the high lipid content present in this residue (23.08 g/100 g DM).
The oil content in the residues varied with the extraction methods used in the fruit during processing in industry. The lowest lipid content was obtained in the powder from pineapple byproducts (1.02 g/100 g DM), followed by the papaya (2.48 g/100 g DM) and blueberry (4.13 g/100 g DM) byproduct powders. These values are in agreement with the quantities present in the powder of other types of fruit byproducts, such as orange (1.83 g/100 g DM), apple (4.46 g/100 g) and mango (2.2 g/100 g DM) (Crizel et al. 2013; Ajila et al. 2008; Figuerola et al. 2005).
The protein content of the pineapple and blueberry byproduct powders (6.72 g/100 g and 7.55 g/100 g DM, respectively) were considered equal and were different from the other samples, while the papaya byproduct powder sample showed the highest protein content (17.72 g/100 g DM). The values obtained in this study were higher than the results reported by López-Marcos et al. (2015) for lemon albedo dietary fiber (5.25 g/100 g DM) and by Martinez et al. (2012) for guava (4.8 g/100 g DM), pineapple (4.0 g/100 g DM) and passion fruit (6.2 g/100 g) co-products.
Dietary fiber has been considered an important nutrient in the human diet. Many researchers have found fruit and vegetable byproducts to be a good source of dietary fiber (O’shea et al. 2012). The pineapple, papaya, blueberry and olive byproduct powders showed total dietary fiber (TDF) content (Table 2) ranging from 32.23 to 53.68 g/100 g DM. These were greater than the TDF content of other fruit fiber sources, such as mango (28.05 g/100 g DM) (Vergara-Valencia et al. 2007), residues of grapefruit juice extraction (44.2 g/100 g DM) (Figuerola et al. 2005), and peach and pear processing byproducts (35.8 g/100 g DM and 36.1 g/100 g DM, respectively) (Grigelmo-Miguel and Martin-Belloso 1999). TDF content of pineapple, papaya, blueberry and olive byproducts similar to the fiber content observed for cereals, such as wheat bran (44%), and their contents were higher than oat bran fiber (23.8%) (Grigelmo-Miguel and Martin-Belloso 1999).
Table 2.
Total dietary fiber (TDF), insoluble dietary fiber (IDF), and soluble dietary fiber (SDF) of pineapple, papaya, blueberry and olive byproduct powders
| Byproduct powder | TDF* | IDF* | SDF* |
|---|---|---|---|
| Pineapple | 45.23 ± 0.35b | 37.58 ± 1.62b | 7.65 ± 1.97a |
| Papaya | 32.23 ± 0.49c | 30.34 ± 0.20c | 1.87 ± 0.29b |
| Blueberry | 47.51 ± 0.05b | 45.95 ± 0.85a | 1.55 ± 0.81b |
| Olive | 53.68 ± 0.97a | 49.34 ± 1.39a | 4.34 ± 0.42ab |
TDF total dietary fiber, IDF insoluble dietary fiber, SDF soluble dietary fiber
* Results are the means of three determinations ± standard deviation. Different letters in the same column are significantly different as determined by Tukey’s test (p ≤ 0.05)
In all of the byproduct powders, the insoluble dietary fiber (IDF) fraction was higher than the soluble dietary fiber (SDF) portion, whereas approximately 90% of the TDF content belonged to the insoluble fraction. These large quantities of IDF in the byproduct powder of these fruits indicated the presence of significant amounts of celluloses and hemicelluloses (Martínez et al. 2012). The advantages of IDF on human health included an increase in satiety and a contribution to the smooth functioning of the intestinal tract (Elleuch et al. 2011).
Due to the insoluble and soluble properties of fiber present in powder, it has a variety of technological attributes, such as gelling, water binding, thickening and structure building, allowing its use as a replacement for fat. Therefore, fiber has been used as an ingredient with specific functions in food production. These properties are important for allowing utilization of the fiber or powder rich in fiber in the fabrication of different foods, such as bakery and meat products, snacks and diabetic beverages (Elleuch et al. 2011; O’shea et al. 2012).
Functional properties
The functional properties of powder, such as its water and oil holding capacities and solubility, are very important because they determine the functionality of the powder in the foods. The papaya powder showed the highest WHC value of 8.93 g water/g powder, followed by pineapple powder (6.06 g water/g powder), and both presented significant differences (p ≤ 0.05) compared to the other powders. Viuda-Martos et al. (2012) analyzed pomegranate whole fruit juice bagasse and obtained WHC values of 4.9 g water/g dry fiber. According López-Marcos et al. (2015) the WHC was associated with dietary fiber properties, which affected metabolic activity of fiber in the gastrointestinal tract.
The incorporation of powder with a high WHC can improve the technological characteristics of the food products, such as decrease the calories, and syneresis, while changing the viscosity and texture of the final product (Grigelmo-Miguel and Martin-Belloso 1999).
The OHC of the powder depends on the chemical and physical structures of the polysaccharides. This property is important to avoid fat loss during the cooking process; consequently, it has an auxiliary use in flavor preservation (Martínez et al. 2012). The values of OHC ranged from 2.7 to 3.81 g oil/g powder, and the highest values were obtained for the pineapple and papaya byproduct powders, followed by blueberry and olive powders. The OHC values of these powders were higher than the results reported by Figuerola et al. (2005) for apple fiber (0.60–1.45 g/g) and grapefruit (1.20–1.52 g/g), as well as the values obtained by Martínez et al. (2012) for mango (1.6 g/g), passion fruit (0.9 g/g) and guava (0.7 g/g) co-products. In addition, the particle size can also influence this capacity, since smaller the particle size, the higher the oil holding capacity because the smaller particles have larger surface areas (Viuda-Martos et al. 2012).
The solubility of the powder is associated with the structure of its polysaccharides, and whereas these compounds are classified as regular (insoluble) or irregular (soluble) according to their structure (Elleuch et al. 2011). The solubilities of the powders (Table 3) ranged from 24.19 to 59.91%, and the highest value was presented by papaya byproduct powder sample, followed by pineapple, blueberry and olive powders, with significant differences (p ≤ 0.05) found among all of the powders. The byproduct powders analyzed in this study presented higher values compared to other powders, such as the fiber in orange byproducts, which demonstrated a solubility value of 28.95% (Crizel et al. 2013).
Table 3.
Technological properties, total phenolic compounds and antioxidant activities of the pineapple, papaya, blueberry and olive byproduct powders
| Byproduct powder | WHC (g water/g powder)* | OHC (g oil/g powder)* | Solubility (%)* | Total phenolic content (mg/g GAE)* | Antioxidant activity (IC 50 in mg powder)* |
|---|---|---|---|---|---|
| Pineapple | 6.06 ± 0.29b | 3.45 ± 0.09a | 55.00 ± 0.13b | 12.28 ± 0.04c | 23.96 ± 1.90a |
| Papaya | 8.93 ± 1.05a | 3.81 ± 0.58a | 59.91 ± 0.23a | 15.35 ± 0.63b | 23.55 ± 0.68a |
| Blueberry | 4.01 ± 0.03c | 3.21 ± 0.16ab | 40.15 ± 0.55c | 23.59 ± 0.85a | 4.62 ± 0.18c |
| Olive | 3.57 ± 0.21c | 2.60 ± 0.08b | 24.19 ± 0.36d | 10.02 ± 0.98c | 19.17 ± 1.01b |
* Results are the means of three determinations ± standard deviation. Different letters in the same column are significantly different as determined by Tukey’s test (p ≤ 0.05)
Total phenolic content
The total phenolic content of the byproduct powders was evaluated and expressed as gallic acid equivalents. The samples evaluated presented values ranging from 10.02 to 23.59 mg GAE/g. The blueberry byproduct powder sample showed the greatest total phenolic content (p ≤ 0.05), followed by papaya powder sample and then the pineapple and olive powders, which were not significantly different from each other. These values are in agreement with data reported in the literature for byproducts of freezer-dried fruits, including guava (19.87 mg GAE/g), soursop (14.39 mg GAE/g), papaya (7.83 mg GAE/g), mango (3.76 mg GAE/g) and passion fruit (4.51 mg GAE/g) (Morais Ribeiro da Silva et al. 2014).
Su and Silva (2006) evaluated the phenolic content using the Folin-Ciocalteu method in blueberry pomace obtained by juice extraction, and the result (20.7 mg GAE/g) was similar to the powder byproducts analyzed in this study.
Antioxidant activity-DPPH method
The antioxidant activity evaluates the capacity of substances to deactivate the DPPH radical by electron transfers. The results of the byproduct powders antioxidant capacities were expressed using the efficient concentration (EC50), which represents the amount of antioxidant necessary to decrease the initial DPPH concentration by 50% (Magalhães et al. 2008).
The DPPH analysis revealed the best results for the blueberry byproduct powder sample, followed by olive, papaya and pineapple powders. Studies reported that high blueberry antioxidant activity might be related to the high content of phenolic compounds because among many fruits and vegetables, blueberries can be considered a major source of antioxidants (Vrhovsek et al. 2012).
A study performed by Guimarães et al. (2010) obtained antioxidant activity EC50 values (mg/mL) lower than that of blueberry powder when assessing the polar fractions of orange and grapefruit peel (4.99 and 5.15 mg/mL, respectively).
The contents and types of phenolic and other antioxidant compounds in fruit byproducts depend on numerous factors, which can include differences in the varieties, maturity and season; environmental aspects, such as the soil type and climate; genetic factors; and the processing method, including the extraction methods and solvent used (Martínez et al. 2012). Factors such as the temperature, time and type of drying also influence the amount of antioxidant compounds in the powder.
Carotenoid identification and quantification
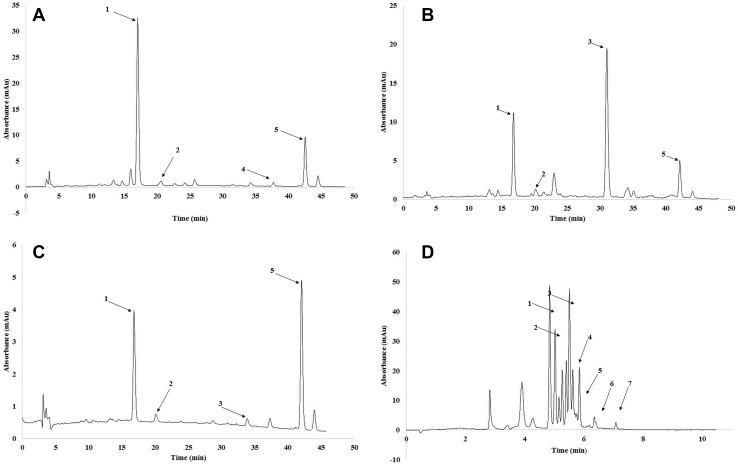
Table 4 shows the carotenoid compositions of the pineapple, papaya and olive byproducts powders (Fig. 1a, b, c). The papaya powder sample showed the highest content of total carotenoids, including lutein, zeaxanthin, cryptoxanthin and β-carotene. Statistical analysis showed that the pineapple and olive byproduct powders did not differ in their carotenoid content but showed a difference in their major carotenoid. Lutein was the main carotenoid in pineapple powder, and pineapple powder was the only one to present α-carotene.
Table 4.
Carotenoid compositions of the pineapple, papaya and olive byproduct powders
| Peak no. | Carotenoids | Retention time (min) | Concentration [mg/100 g (DM)]* Byproduct powder |
||
|---|---|---|---|---|---|
| Pineapple | Papaya | Olive | |||
| 1 | Lutein | 17.790 | 1.12 ± 0.17b | 2.41 ± 0.05a | 0.26 ± 0.03c |
| 2 | Zeaxanthin | 20.746 | 0.10 ± 0.02b | 0.73 ± 0.08a | 0.10 ± 0.001b |
| 3 | Cryptoxanthin | 31.212 | ND | 9.21 ± 0.31a | 0.34 ± 0.01b |
| 4 | α-Carotene | 37.746 | 0.058 ± 0.003 | ND | ND |
| 5 | β-Carotene | 42.203 | 0.75 ± 0.04b | 3.21 ± 0.36a | 0.94 ± 0.07b |
| Total carotenoids | 2.02 ± 0.13b | 15.56 ± 0.35a | 1.64 ± 0.06b | ||
ND not detected
* Results are the means of three determinations ± standard deviation. Different letters in the same line are significantly different as determined by Tukey’s test (p ≤ 0.05)
Fig. 1.
Chromatograms, obtained by HPLC, for carotenoids from byproduct powder pineapple (a), papaya (b) and olive (c) and of anthocyanins from byproduct powder blueberry (d)
The pineapple, papaya and olive byproducts powders showed total carotenoid content higher than those obtained by Crizel et al. (2013) for orange byproduct fiber, including F1 (peel, bagasse and seed fiber) and F2 (peel fiber) with contents of 0.95 mg/100 g and 1.21 mg/100 g, respectively. The carotenoid contents obtained in this study were also higher than those for avocado peel (1.52 mg/100 g) and banana peel (0.4 mg/100 g) (Wang et al. 2010).
Sentanin and Amaya (2007) evaluated the carotenoid levels in formosa papaya determined by HPLC and obtained lower values than the cryptoxanthin and β-carotene contents (7.0 and 1.2 μg/g, respectively) found in the powder of papaya byproducts because carotenoids are usually more concentrated in the peels than in the pulp of the fruits. Geographical effects also influenced the level of carotenoids relative to the soil and climate where the fruits were produced because exposure to sunlight and elevated temperatures increases carotenoid biosynthesis in fruits (Rodriguez-Amaya 2001).
Morais Ribeiro da Silva et al. (2014) quantified the β-carotene in freeze-dried byproducts of tropical fruits, including pineapple (peels and pulp leftovers) and papaya (peels, pulp leftovers, and seeds) and obtained contents of 0.16 mg/100 g and 0.49 mg/100 g, respectively. These results were lower than those demonstrated in this study, which can be explained by differences in the compositions of the byproducts as well as by the type, temperature and time of drying, processing method and solvent used in the extraction and particle size.
Anthocyanins identification and quantification
Table 5 shows the results of the anthocyanins obtained and quantitatively analyzed in the powder of blueberry byproducts (Fig. 1d).
Table 5.
Anthocyanins of blueberry byproducts powder and methanol:acetone extracts
| Peak | Anthocyanin | Retention time (min) | Blueberry byproducts powder Anthocyanins content (mg/100 g)* |
|---|---|---|---|
| 1 | Delphinidin 3-glucoside | 4.45 | 824.9 ± 11.9a |
| 2 | Cyanidin 3-glucoside | 4.89 | 303 ± 7.4a |
| 3 | Malvidin 3-glucoside | 5.68 | 513.2 ± 13a |
| 4 | Delphinidin aglycone | 5.76 | 47.8 ± 5a |
| 5 | Pelargonidin 3-glucoside | 5.92 | 222.7 ± 9.8a |
| 6 | Cyanidin aglycone | 6.38 | 112.8 ± 0.5 |
| 7 | Malvidin aglycone | 7.09 | 38.9 ± 0.2 |
| Total anthocyanins | 2063.4 ± 17a | ||
ND not detected
* Results are the means of three determinations ± standard deviation. Different letters in the same line are significantly different as determined by Tukey test (p ≤ 0.05)
The blueberry byproduct powder exhibited a high anthocyanin content of 2063.4 mg/100 g, with delphinidin 3-glucoside being the anthocyanin found in the greatest amount (824.9 mg/100 g), followed malvidin 3-glucoside, cyaniding 3-glucoside, pelargonidin 3-glucoside, cyaniding aglycone, malvidin aglycone and delphinidin aglycone. Reque et al. (2014) identified delphinidin 3-glucoside and malvidin 3-glucoside as the major anthocyanins in blueberry fruit.
The high anthocyanin content in blueberry powder justified its high Folin reduction capability and high ability to disable the DPPH radical, which were superior to the other powders analyzed in this study.
The determination of the total anthocyanins of a frozen rabbiteye blueberry pomace sample obtained by processing juice was performed by Su and Silva (2006), showing an 11.9 mg/g DM total anthocyanin content using a pH differential method. The result obtained in this study (20.63 mg/g DM) was higher than the results reported by Su and Silva; many factors can be responsible for this difference. The anthocyanin content can vary according to cultivars species season and growing location of different methods and the solvents used, all of which may contribute to the variance in the reported levels of anthocyanins, phenolics, and antioxidants (Moyer et al. 2002). According to Nicoue et al. (2007) reported the value of total anthocyanins measured by HPLC–DAD, were lower values than analysis by the pH differential method. The powder drying process also influenced the total anthocyanins, particularly at high temperatures and for long periods because the process can destroy phenolic compounds. Studies show that the drying temperature suitable for maintaining the antioxidant activities of products is 60 °C, suggesting that antioxidant compounds have a higher resistance to heat degradation (Patras et al. 2010).
Due to the antioxidant profile of the powder from blueberry byproducts, an interesting use for it could be as an ingredient in the development of functional foods. The application of byproducts in the production of powder makes their use even more interesting because of their low economic value and because they are environmentally friendly.
Conclusion
The results confirm the high potential for the recovery of waste derived from the fruit processing industry to obtain dietary powder.
The powders showed good functional properties, especially the papaya byproduct powder sample, which showed the highest WHC, OHC and solubility values, thus enabling its use to improve the texture and reduce the calories in food. All of the powders exhibited high TDF content in their composition, high Folin reduction capacities and good abilities to disable the DPPH radical, with the blueberry byproduct powder sample obtaining the highest values for these properties and the highest concentration of anthocyanins. The carotenoid content was significant in papaya powder, followed by the pineapple and olive byproduct powders compared to other plant sources.
The use of these byproducts maybe interesting and viable in the food industry due to their low cost, and nutritional properties related to human.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Tainara de Moraes Crizel, Email: tainara_mc@hotmail.com.
Vanessa Stahl Hermes, Email: van.hermes@gmail.com.
Alessandro de Oliveira Rios, Email: alessandro.rios@ufrgs.br.
Simone Hickmann Flôres, Phone: +555133089789, Email: simone.flores@ufrgs.br.
References
- Ajila CM, Leelavathi K, Rao UJSP. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J Cereal Sci. 2008;48:319–326. doi: 10.1016/j.jcs.2007.10.001. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Arlington: Association of Official Analytical Chemist; 1990. [Google Scholar]
- Ayala-Zavala JF, Vega-Vega V, Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodriguez JA, Siddiqui MW, Dávila-Aviña JE, González-Aguilar GA. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res Int. 2011;44:1866–1874. doi: 10.1016/j.foodres.2011.02.021. [DOI] [Google Scholar]
- Bhol S, Lanka D, Bosco SJD. Quality characteristics and antioxidant properties of breads incorporated with pomegranate whole fruit bagasse. J Food Sci Technol. 2015;53:1717–1721. doi: 10.1007/s13197-015-2085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Technol. 2005;6:420–428. doi: 10.1016/j.ifset.2005.05.003. [DOI] [Google Scholar]
- Crizel TM, Jablonski A, Rios AO, Rech R, Flôres SH. Dietary fiber from orange byproducts as a potential fat replacer. LWT Food Sci Technol. 2013;53:9–14. doi: 10.1016/j.lwt.2013.02.002. [DOI] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;122:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- Fernández-López J, Sendra-Nadal E, Navarro C, Sayas E, Viuda-Martos M, Alvarez JAP. Storage stability of a high dietary fibre powder from orange by-products. Int J Food Sci Technol. 2009;44:748–756. doi: 10.1111/j.1365-2621.2008.01892.x. [DOI] [Google Scholar]
- Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Martin-Belloso O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT Food Sci Technol. 1999;32:503–508. doi: 10.1006/fstl.1999.0587. [DOI] [Google Scholar]
- Guimarães R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM, Ferreira ICFR. Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem Toxicol. 2010;48:99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Lima H, Corrêa NCF, Santos O, Lourenço LFH. Use of agroindustrial wastes (açai fiber and glycerol) in the preparation of cookies. J Food Sci Technol. 2014;52:4593–4599. doi: 10.1007/s13197-014-1493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Marcos MC, Bailina C, Viuda-Martos M, Pérez-Alvarez JA, Fernández-López J. Properties of dietary fibers from agroindustrial coproducts as source for fiber-enriched foods. Food Bioprocess Technol. 2015;8:2400–2408. doi: 10.1007/s11947-015-1591-z. [DOI] [Google Scholar]
- Magalhães LM, Segundo MA, Reis S, Lima JLFC. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613:1–19. doi: 10.1016/j.aca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Martínez R, Torres P, Meneses MA, Figueroa JG, Pérez-Álvarez JA, Viuda-Martos M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012;135:1520–1526. doi: 10.1016/j.foodchem.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Mercadante AZ, Britton G, Rodriguez-Amaya DB. Carotenoids from yellow Passion fruit (Passiflora edulis) J Agric Food Chem. 1998;46:4102–4106. doi: 10.1021/jf9801724. [DOI] [Google Scholar]
- Morais Ribeiro da Silva L, Teixeira de Figueiredo EA, Pontes Silva Ricardo NM, Gusmao Pinto Vieira I, Wilane de Figueiredo R, Montenegro Brasil I, Gomes CL. Quantification of bioactive compounds in pulps and byproducts of tropical fruits from Brazil. Food Chem. 2014;143:389–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J Agric Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- Nicoue EE, Savard S, Belkacemi K. Anthocyanins in wild blueberries of Quebec: extraction and identification. J Agric Food Chem. 2007;55:5626–5635. doi: 10.1021/jf0703304. [DOI] [PubMed] [Google Scholar]
- O’shea N, Arendt EK, Gallagher E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov Food Sci Emerg Technol. 2012;16:1–10. doi: 10.1016/j.ifset.2012.06.002. [DOI] [Google Scholar]
- Patras A, Brunton NP, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- Reque PM, Steffens RS, Jablonski A, Flôres SH, Rios AO, Jong EV. Cold storage of blueberry (Vaccinium spp.) fruits and juice: anthocyanin stability and antioxidant activity. J Food Compos Anal. 2014;33:111–116. doi: 10.1016/j.jfca.2013.11.007. [DOI] [Google Scholar]
- Rodrigues E, Poerner N, Rockenbach II, Gonzaga LV, Mendes CR, Fett R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Ciênc Tecnol Aliment. 2014;31:911–917. [Google Scholar]
- Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. Washington: International Life Sciences Institute Press; 2001. [Google Scholar]
- Saikia S, Mahanta CL. In vitro physicochemical, phytochemical and functional properties of fiber rich fractions derived from by-products of six fruits. J Food Sci Technol. 2015;53:1496–1504. doi: 10.1007/s13197-015-2120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentanin MA, Amaya DBR. Teores de carotenoides em mamão e pêssego determinados por cromatografia líquida de alta eficiência. Ciênc Tecnol Aliment. 2007;27:13–19. doi: 10.1590/S0101-20612007000100003. [DOI] [Google Scholar]
- Singh JP, Kaur A, Singh N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J Food Sci Technol. 2015;53:1269–1278. doi: 10.1007/s13197-015-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Su M, Silva JL. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006;97:447–451. doi: 10.1016/j.foodchem.2005.05.023. [DOI] [Google Scholar]
- Tarazona-Díaz MP, Aguayo E. Assessment of by-products from fresh-cut products for reuse as bioactive compounds. Food Sci Technol Int. 2013;19:439–446. doi: 10.1177/1082013212455346. [DOI] [PubMed] [Google Scholar]
- Vergara-Valencia N, Granados-Pérez E, Agama-Acevedo E, Tovar J, Ruales J, Bello-Pérez LA. Fibre concentrate from mango fruit: characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT Food Sci Technol. 2007;40:722–729. doi: 10.1016/j.lwt.2006.02.028. [DOI] [Google Scholar]
- Viuda-Martos M, Sánchez-Zapata E, Martín-Sánchez AM, Ruiz-Navajas Y, Fernández-López J, Sendra E, Pérez-Álvarez JA, Sayas E, Navarro C. Technological properties of pomegranate (Punica granatum L.) peel extract obtained as co-product in the juice processing. In: Cho S, Almeida N, editors. Dietary fiber and health, chap 31. Boca Raton: CRC Press; 2012. pp. 443–452. [Google Scholar]
- Vrhovsek U, Masuero D, Palmieri L, Mattivi F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J Food Compos Anal. 2012;25:9–16. doi: 10.1016/j.jfca.2011.04.015. [DOI] [Google Scholar]
- Wang W, Bostic TR, Gu L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010;122:1193–1198. doi: 10.1016/j.foodchem.2010.03.114. [DOI] [Google Scholar]
- Zanatta CF, Mercadante AZ. Carotenoid composition from the Brazilian tropical fruit camu-camu (Myrciaria dubia) Food Chem. 2007;101:1526–1532. doi: 10.1016/j.foodchem.2006.04.004. [DOI] [Google Scholar]
- Zanatta CF, Cuevas E, Bobbio FO, Winterhalter P, Mercadante AZ. Determination of anthocyanins from camu-camu (Myrciaria dubia) by HPLC–PDA, HPLC–MS and NMR. J Agric Food Chem. 2005;53:9531–9535. doi: 10.1021/jf051357v. [DOI] [PubMed] [Google Scholar]