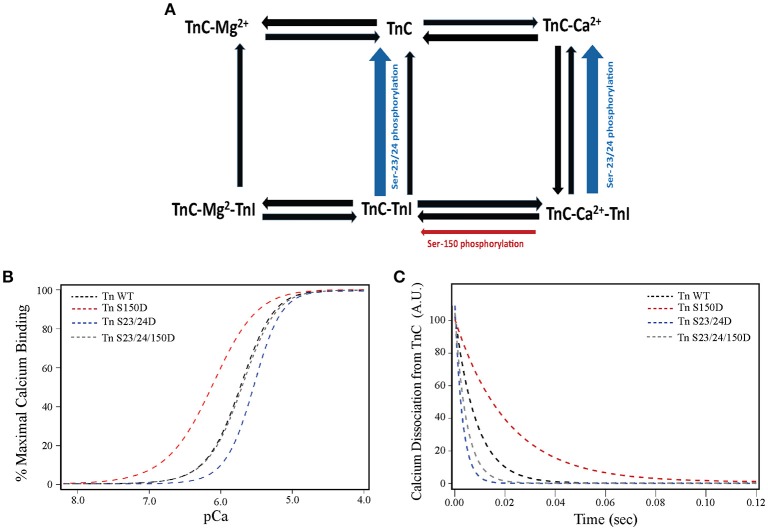
Figure 1.
TnI Ser-23/24 and Ser-150 differentially alter thin filament activation through different TnI mediated mechanisms. (A) Diagrammatic representation of the biochemical states in the Siddiqui et al. model (Siddiqui et al., 2016). The rates altered to fit our previous biochemical data for thin filaments containing TnI Ser-150 (red arrow) and Ser-23/24 phosphorylation (blue arrow) are identified. Model simulation of (B) steady-state Ca2+-binding and (C) Ca2+ dissociation from TnC in human thin filaments containing WT (Tn WT, black dashed line), Ser-150 pseudo-phosphorylated (Tn S150D, red dashed line), Ser-23/24 pseudo-phosphorylated (Tn S23/24D, blue dashed line) or Ser-23/24 and Ser-150 pseudo-phosphorylated (Tn S23/24/150D, gray dashed line) TnI demonstrating a similar effect of these phosphorylations to our previously reported biochemical measurements (Nixon et al., 2014).