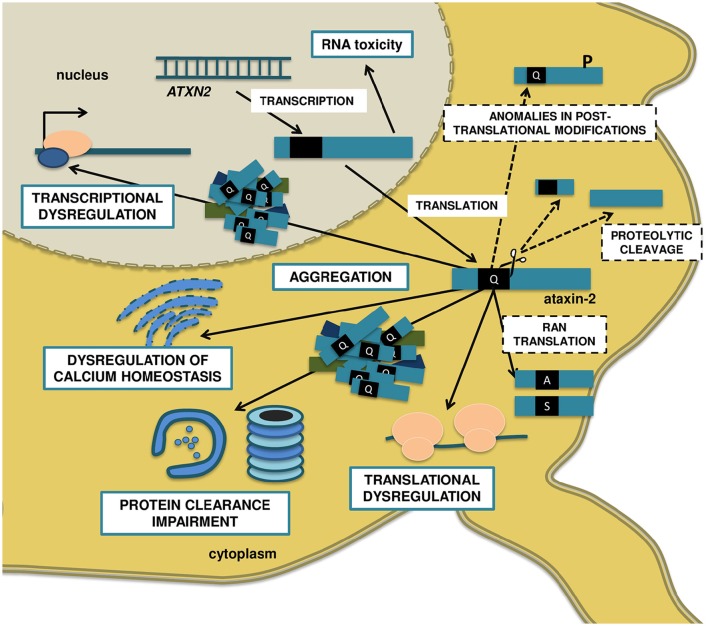
Figure 1.
Molecular mechanisms proposed to be involved in SCA2 pathogenesis. The anti-sense transcription of the ATXN2 gene gives origin to the repeat-expanded ATXN2-AS, with the ability to form hairpin structures and induce toxicity. The sense transcription encodes to the polyQ-expanded ataxin-2 protein, which assembles in insoluble, not always ubiquitinated cytoplasmic aggregates, and recruits other proteins like E3 ubiquitin ligases. Ultimately, the UPS might get overburden, disturbing the neuronal protein turnover and engaging in aberrant proteolytic cleavage, with the formation of N-terminal PolyQ-containing toxic fragments. Also, the mutated ataxin-2 decreases the ability of WT ataxin-2 to stabilize mRNAs and upregulate protein expression, resulting in transcriptional and translational dysregulations. On the other hand, the expanded polyQ protein binds to receptors in the ER and promotes a significant increase in intracellular calcium and, consequently, excitotoxicity, and enhanced LTD in cerebellar PCs. Finally, posttranslational modifications such as phosphorylation at specific residues might modulate the toxicity of ataxin-2, and RAN translation can result in additional polyalanine and/or polyserine toxic proteins. Blue, solid line boxes represent well-studied disease mechanisms, with extensive supporting evidence in SCA2. Black, dashed line boxes represent pathogenic mechanisms that are well established in other PolyQ disorders like HD and other SCAs, but whose relevance to SCA2 is still unclear.