Abstract
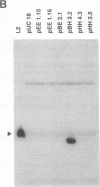
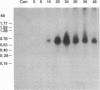
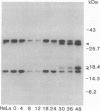
Chlamydiae are prokaryotic obligate intracellular parasites that undergo a biphasic life cycle involving an infectious, extracellular form known as elementary bodies and an intracellular, replicating form termed reticulate bodies. We have purified from Chlamydia trachomatis a very basic elementary body-specific protein with an apparent molecular mass of 18 kDa, determined its N-terminal amino acid sequence, and cloned the encoding gene. Sequence analysis of the cloned gene revealed some remarkable properties for its expressed product, including a high lysine content (29%), a correspondingly high pI, and significant homology to the H1 class of eukaryotic histones. Furthermore, a monoclonal antibody to this chlamydial histone analog, termed Hc1, displayed immunoblot and antinuclear specificity suggestive of cross-reactivity to H1 histones. The gene was expressed only during the late stages of the chlamydial life cycle concomitant with the reorganization of chlamydial reticulate bodies into elementary bodies, suggesting that the Hc1 protein plays a role in the condensation of chlamydial chromatin during intracellular differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Mitchell T., Harborne N., Bohm L., Crane-Robinson C. Roles of H1 domains in determining higher order chromatin structure and H1 location. J Mol Biol. 1986 Feb 20;187(4):591–601. doi: 10.1016/0022-2836(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove L., Alderuccio F., Toh B. H., Pedersen J., Holliday A., McKenzie I. A monoclonal antibody to the surface membrane of human platelets which inhibits ristocetin- and collagen-induced platelet aggregation reacts with H1 histones of cell nuclei. Clin Exp Immunol. 1987 Aug;69(2):409–419. [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Poffenroth L., Wilt J. C., Kordová N. Ultrastructural studies of the nucleoids of the pleomorphic forms of Chlamydia psittaci 6BC: a comparison with bacteria. Can J Microbiol. 1976 Jan;22(1):16–28. doi: 10.1139/m76-003. [DOI] [PubMed] [Google Scholar]
- Deretic V., Konyecsni W. M. A procaryotic regulatory factor with a histone H1-like carboxy-terminal domain: clonal variation of repeats within algP, a gene involved in regulation of mucoidy in Pseudomonas aeruginosa. J Bacteriol. 1990 Oct;172(10):5544–5554. doi: 10.1128/jb.172.10.5544-5554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousson S., Gorka C., Gilly C., Lawrence J. J. Histone H1(0) mapping using monoclonal antibodies. Eur J Immunol. 1989 Jun;19(6):1123–1129. doi: 10.1002/eji.1830190624. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Miceli M., Sublett J. E. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1986 Feb;165(2):379–385. doi: 10.1128/jb.165.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Kato J., Misra T. K., Chakrabarty A. M. AlgR3, a protein resembling eukaryotic histone H1, regulates alginate synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2887–2891. doi: 10.1073/pnas.87.8.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Wyrick P. B., Manire G. P. Effect of alkali on the structure of cell envelopes of Chlamydia psittaci elementary bodies. J Bacteriol. 1976 Jan;125(1):300–307. doi: 10.1128/jb.125.1.300-307.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M. A., Marzluff W. F. Structure of an unusual sea urchin U1 RNA gene cluster. Gene. 1988 Apr 15;64(1):53–63. doi: 10.1016/0378-1119(88)90480-5. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J Biol Chem. 1971 Dec 25;246(24):7557–7560. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia L. M., Engel J. N., Ganem D. Chlamydial gene encoding a 70-kilodalton antigen in Escherichia coli: analysis of expression signals and identification of the gene product. J Bacteriol. 1989 Jan;171(1):335–341. doi: 10.1128/jb.171.1.335-341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988 Feb;170(2):744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Stephens R. S. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988 Jul;56(7):1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Manning D. S., Caldwell H. D. Multiple tandem promoters of the major outer membrane protein gene (omp1) of Chlamydia psittaci. Infect Immun. 1990 Sep;58(9):2850–2855. doi: 10.1128/iai.58.9.2850-2855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]