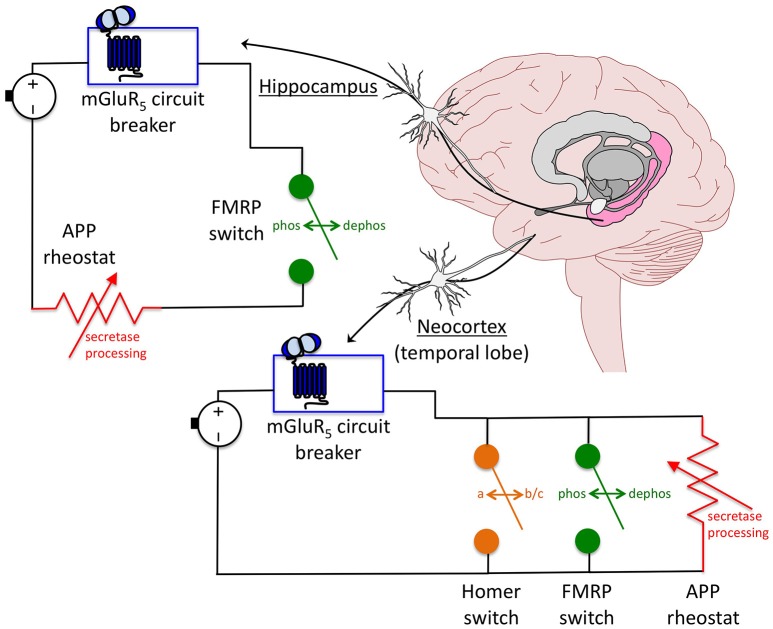
Figure 2.
Model for an APP-induced short circuit in FXS. APP acts as a rheostat (i.e., variable resistor, dimmer switch) in a circuit where mGluR5 inhibitors are a circuit breaker and FMRP is an automatic transfer switch that regulate neuronal excitability. The FMRP switch is dependent on a rapid dephosphorylation reaction in response to mGluR5 activation. In the absence of FMRP, the circuit is constitutively on. In the presence of mGluR5 inhibitors, the circuit is shut down. The downstream APP circuitry appears to be wired differently dependent on brain region. In the neocortex, knockdown of individual proteins including mGluR5, Homer and APP completely rescues excitability levels suggesting that these components are arranged in a parallel circuit whereby there is more than one continuous signaling pathway between mGluR5 activation and excitability output. Rescue of any one of the parallel components is sufficient to restore synaptic homeostasis. In the hippocampus, ictal burst duration, but not induction, is rescued in Fmr1KO/AppHET slices in response to DHPG treatment. This incomplete rescue suggests that APP and FMRP are wired in series downstream of mGluR5, and that the APP rheostat is overloaded in the absence of FMRP resulting in a short circuit. This model has important implications for therapeutic development and suggests that APP should be considered as a drug target for FXS as part of a multi-drug therapeutic strategy.