Abstract
Introduction
To study the presence of European bat lyssavirus (EBLV) infections in bat reservoirs in Sweden, active surveillance was performed during the summers from 2008 to 2013.
Material and methods
Bat specimens were collected at >20 bat colonies in the central, southeastern, and southern parts of Sweden. In total, blood and saliva of 452 bats were examined by a virus neutralization test and by reverse transcription polymerase chain reactions (RT-PCRs).
Results and discussion
EBLV neutralizing antibodies were detected in 14 Daubenton's bats (Myotis daubentonii), all trapped in Skåne or Småland (south and southeast of Sweden). The result was not unexpected since EBLV has been shown to be present in many neighboring countries, for example, Denmark, Finland, Germany, and Norway. However, Sweden has been regarded free of rabies in terrestrial mammals since 1896. Although very rare, spillover of EBLV into other animals and humans have occurred, and the risk of EBLV infection to other species including humans should not be ignored. This is the first report of lyssavirus infection in Swedish bats.
Keywords: bat, Lyssavirus, rabies, EBLV, Myotis daubentonii, Daubenton's bat, Northern bat
Rabies is a viral zoonotic disease that is fatal in humans and other mammals once clinical signs develop. The disease is caused by different lyssavirus species of the family Rhabdoviridae (1). The genus Lyssavirus can be divided into 14 currently described taxonomic species, which, according to phylogenetic analyses, all probably originated in bats (2). In addition, Lleida bat lyssavirus (LLEBV) originally found in Spain in 2012 has been proposed as a new species (3).
Phylogroup I comprises the classical rabies virus (RABV) and the majority of the bat lyssaviruses, whereas Lagos bat virus, Shimoni bat virus, and Mokola virus form phylogroup II (4, 5). West Caucasian bat virus (WCBV), Ikoma lyssavirus, and LLEBV may represent a possible new phylogroup (2).
Today, bat rabies in Europe is known to be caused by five lyssaviruses: European bat lyssavirus type 1 (EBLV-1) and type 2 (EBLV-2), WCBV, LLEBV, and Bokeloh bat lyssavirus (3, 6). EBLV-1 and EBLV-2, the two lyssaviruses mainly found, are also designated as genotypes (or species) 5 and 6, respectively (7).
Since the first reported case of bat rabies in Germany in 1954, 1,064 rabies cases have been reported in 11 of the 45 known indigenous bat species in 16 European countries (8). EBLV-1 seems to be mainly associated with infection of serotine bats (Eptesicus serotinus) (9), although it has occasionally been reported also as ‘spillovers’ in incidental hosts (10–12). Compared to EBLV-1, EBLV-2 has been reported at much fewer occasions, around 20, at present reported from Switzerland, Netherlands, UK, Germany, Finland, and Norway (13–15). EBLV-2 has mainly been isolated both from Daubenton's bats (Myotis daubentonii) and pond bats (Myotis dasycneme) (16). Although transmissions of EBLV to terrestrial animals and humans have been shown, the risk of spillover infection is regarded as extremely rare.
A bite of an EBLV-infected bat may, however, result in fatal disease in humans; four fatal human cases have been reported until now. Three of these four infecting viruses were undoubtedly classified as EBLV (9). The most recently reported case was a 56-year-old man, working with bats in Scotland who thought he had been bitten in the hand by a Daubenton's bat (17). Therefore, bat rabies is considered a public health threat in countries where EBLV is endemic in bats.
The first case of bat rabies in Europe was reported in 1954. Approximately 30 years later, in 1985, bat rabies was discovered in Denmark (18).
The same year, a Swiss bat scientist, who at several occasions had been bitten by bats in different countries and the last time in Finland, died from rabies 51 days after the last known bite (19). These two incidents became the starting point for passive surveillance of bat lyssavirus infections in Sweden. All of the investigated Swedish bats have been found grounded (unable to fly or dead) and sent to the laboratory by the public, without any knowledge about the cause of death. All samples were investigated by fluorescent antibody test (20), and until to date, all have been found negative.
Although much more ‘resource-consuming’ compared to passive surveillance, several reports (21–23) have proved the benefit of active surveillance in order to monitor EBLV infection in bats. Active surveillance of bat lyssavirus is based on detection of virus in saliva and/or antibodies in serum of free-living bats.
In 2008, the Swedish Institute for Infectious Disease Control (SMI) and the National Veterinary Institute (SVA) initiated an active surveillance study. The project was continued during the summers of 2009–2013. The study approach was based on the recommendations of the First International Conference ‘Rabies in Europe’, Kiev, Ukraine, 2005.
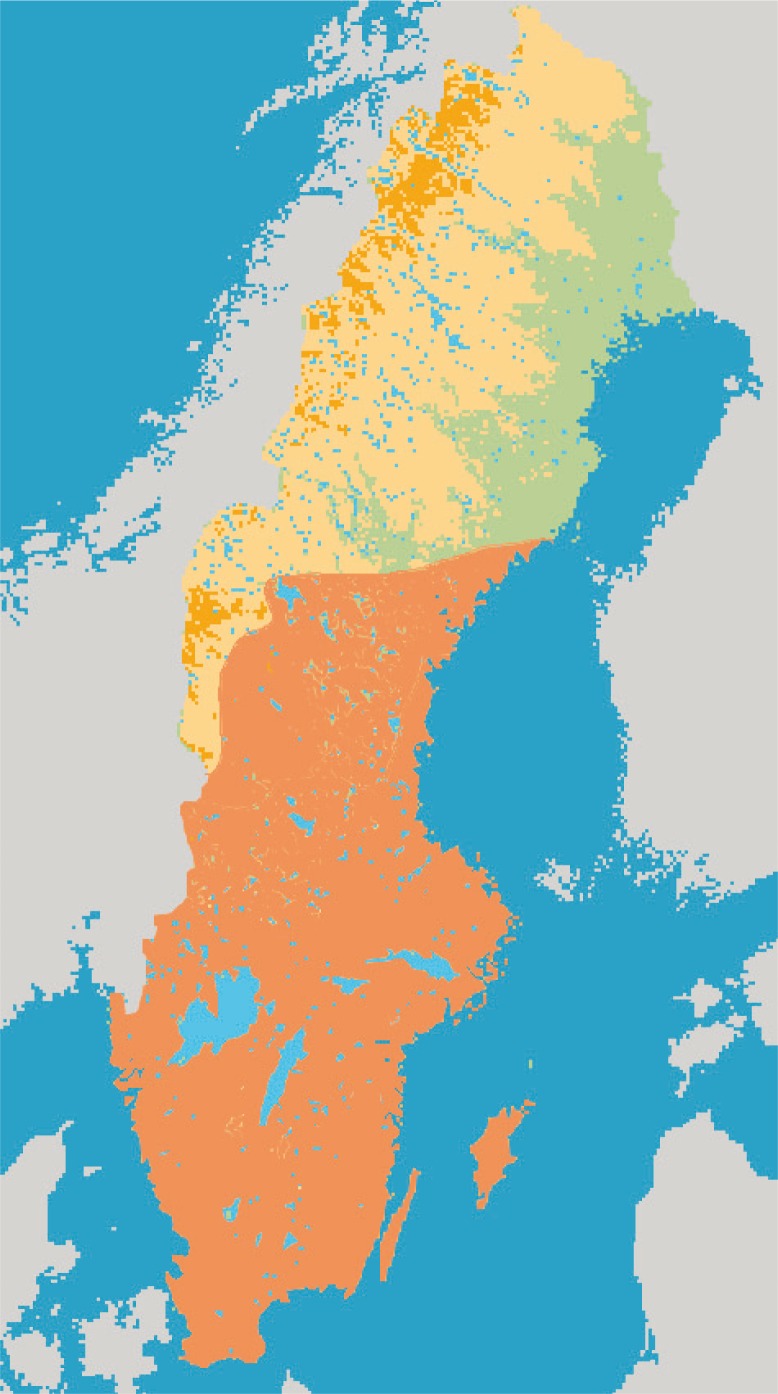
There are 19 species of bats known to be present in Sweden, including the four species – the serotine bat, the Nathusius’ bat, the pond bat, and the Daubenton's bat – that have been associated with EBLV infections in Europe. The serotine bat and the pond bat are relatively uncommon, while Daubenton's bat is frequently found in most parts of Sweden (Fig. 1) and the Nathusius’ bat, which used to be rarely found in Sweden, seems to increase and be spreading north, at least up to Uppland (central Sweden).
Fig. 1.
Distribution of Daubenton's bat in Sweden (area indicated by orange).
In this study, we assessed the prevalence of EBLV-infected Swedish bats in order to obtain an improved understanding of the public health risk that these bats pose. The active samplings were focused on the central, southern, and southeastern parts of Sweden.
Materials and methods
Bat trapping and sample collection
During the summers of 2008, 2009, 2010, 2011, 2012, and 2013, a total of 452 (54, 116, 86, 90, 68, and 38, respectively) bats were captured and sampled in the southern (Skåne, 2008, 2009 and 2013), central (Uppland, 2008, 2009, 2010 and 2011), and southeastern (Småland, 2012) parts of Sweden (Table 1, Fig. 1).
Table 1.
Bat sampling in central Sweden (Uppland), southern Sweden (Skåne), and southeastern Sweden (Småland)
| Region | Year | No. sampling sites | Species (n) |
|---|---|---|---|
| Uppland | 2008 | 2 | Myotis daubentonii (22) |
| Uppland | 2009 | 4 | Eptesicus nilssonii (47) |
| Skåne | 2008 | 4 | Myotis daubentonii (23) |
| Pipistrellus nathusii (4) | |||
| Pipistrellus pygmaeus (2) | |||
| Plecotus auritus (2) | |||
| Eptesicus nilssonii (1) | |||
| Skåne | 2009 | 5 | Myotis daubentonii (22) |
| Uppland | 2011 | 2 | Myotis daubentonii (86) |
| Uppland | 2012 | 3 | Myotis daubentonii (90) |
| Skåne | 2013 | 6 | Myotis daubentonii (37) |
| Plecotus auritus (1) | |||
| Småland | 2012 | 6 | Myotis daubentonii (68) |
The number of captured specimens per species (n) is shown in brackets.
To minimize possible effects on the captured bats’ breeding success, the timing of the sampling was set to mid-July, a time when females and juveniles have left the breeding colonies. The diurnal captures took place from sunset until approximately midnight when most bats pause foraging excursions. Sites for trapping were explored by use of bat detectors at night, or in daytime determined from site characteristics regarding probability of bats’ utilization on foraging excursions.
Bats were captured in standard bat mist nets (dimensions: 2.6−9×2.6 m) that were mounted on 3 m mist net poles (Alana Ecology Ltd., Shropshire, UK) or for the purpose modified and shortened 8 m take-apart angling poles (Fladen Fishing AB, Varberg, Sweden). Mist nets were kept under constant surveillance, and bats were disentangled immediately after capture. Captured bats were individually tagged to avoid repeated sampling if recaptured. Recaptured specimens were immediately released. Bats were then placed individually in lightweight cotton holding bags and, for comfort, kept on a hot-water bottle approximately 30 min to allow for fecal sampling and the bats to calm down. Bat trapping was approved by the Swedish Environmental Protection Agency (Dnr 412-4135-09), and handling (bleeding etc.) was ethically approved by Uppsala Ethical Committee on Animal Experiments (Dnr C175/8).
Collection of specimens
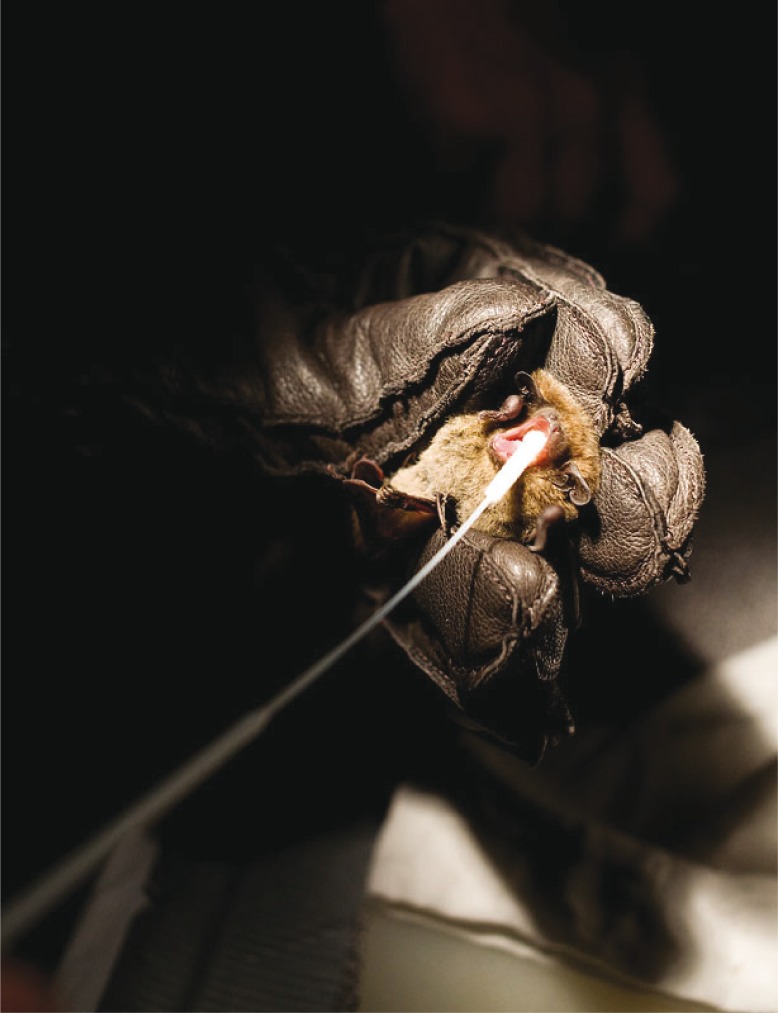
Saliva specimens were collected by a flocked swab (Copan Italia SpA, Via F. Perotti 10 Brescia, Italy) (Fig. 2). After the sampling, the swabs were immediately placed in tubes containing 200–300 µL of Universal Transport Medium (Copan Italia SpA, Via F. Perotti 10 Brescia, Italy). The specimens were kept on dry ice until the day after when they were stored at −70°C.
Fig. 2.
Sampling of a Daubenton's bat. Saliva specimens were collected by a flocked swab.
Blood samples were taken from the tail membrane-vein by a syringe and stored on Nobuto blood filter strips (Advantec, Toyo Roshi Kaisha Ltd, Japan) for analysis. A total volume of 100 µL blood (corresponding to approximately 40 µL serum) was collected. The strips were kept at +4°C until analyzed.
RNA extraction
The samples containing oral swabs and transport medium were vortexed and incubated at room temperature for 15 min prior to extraction. The extractions were performed by using the commercial kit QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions. Extracted RNA was eluted in a final volume of 60 µL. A part of the purified RNA was used immediately, and the remaining part was frozen and stored at −70°C. Bat CNS tissue samples were extracted to use as positive host mRNA control. Cultured EBLV-1 Ra 20/98 (bat isolate) was extracted for use as positive lyssavirus control.
Detection of EBLV RNA
Detection of rabies RNA was performed by a pan-lyssa hemi-nested PCR. The protocol used was a slightly modified version of a previously published protocol by Heaton et al. (24). To confirm the quality of the RNA, 170 of the samples were tested for β-actin mRNA by a real-time, TaqMan RT-PCR as described earlier (25). The same 170 samples were further tested for the presence of lyssavirus RNA as described earlier (25).
Antibody analyses
Blood samples from filter paper were tested for neutralizing antibodies against EBLV-1 (strain EBL Ra 20/98, isolated from a bat trapped in Denmark) or EBLV-2 (strain RV1787 isolated in the UK) by fluorescent antibody virus neutralization (FAVN) as previously described (OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals). The samples were serially diluted at 1:30, 1:90, 1:270, and 1:810. Virus was diluted at 1:200. During 2008–2011, EBLV-1 was used as antigen, while EBLV-2 was used on the samples from 2012 to 2013. EBLV-2 (not available before 2013) was kindly provided by the OIE reference laboratory ANSES (BP 40009, 54220, Malzéville, France). Fluorescein isothiocyanate-conjugated (FITC) anti-rabies virus monoclonal globulin (Fujirebio Diagnostics), diluted 1:50, was used as a conjugate. For the 2008–2010 samples, the OIE dog reference serum was included as a control; from 2011, an in-house control from a dog and a dog control serum from WHO were used.
Due to the most limited volumes of the bat samples, they were initially analyzed in duplicates. However, all positive samples were confirmed by an additional test (in duplicates).
Results
Trapping of bats
To facilitate the mounting of mist nets in perceived bat flyways, most sampling sites were situated under humpback bridges or similar constructions by streams. As a result, there was also a conscious bias in species sampled, since most nets were mounted where almost exclusively M. daubentonii had their flyways.
A total of 452 bats from five different species were captured. In 2008, a total of 54 bats were caught (22 in Uppland and 32 in Skåne); in 2009, 116 bats were caught (47 in Uppland and 69 in Skåne); in 2010 and 2011, 86 and 90 bats, respectively, were caught in Uppland; in 2012, 68 bats were caught in Småland; and in 2013, 38 bats were caught in Skåne (Table 1). The majority of the trapped bats (377) were Daubenton's bats, 52 were Northern bats (Eptesicus nilssonii), and only a few bats were of the species Pipistrellus nathusii (Nathusius’ pipistrelle bat), Plecotus auritus (brown long-eared bat), and Pipistrellus pygmaeus (soprano pipistrelle bat). All the bats were successfully released after sample collections.
Detection of lyssavirus RNA
All 452 oral swabs were found negative for EBLV-1/EBLV-2 RNA when analyzed by the hemi-nested PCR. To confirm the quality of the RNA from the oral swabs, 170 extractions were tested for β-actin mRNA by a real-time RT-PCR, and all samples were found to be positive. The same 170 samples were further examined by a lyssavirus real-time RT-PCR, and all were found negative.
Antibody analyses
Blood samples from a total of 452 bats were analyzed for neutralizing antibodies to EBLV by FAVN. In total, 16 bats, all of Daubenton's bat, were shown to have detectable levels of neutralizing antibodies against EBLV. Of these bats, 14 showed levels of >0.5 IE/mL, which represent a significant antibody response according to WHO/OIE guidelines (Table 2). The sera tested in 2009 (eight positive bats) were tested against EBLV-1, and the sera tested in 2012 (six positive bats) were tested against EBLV-2.
Table 2.
Locations, species, and specimens demography of 14 EBLV antibody positive bats captured
| Region/year | Location | Species | Sex | Age |
|---|---|---|---|---|
| Skåne/2009 | Svenstorp | Myotis daubentonii | female | adult |
| Skåne/2009 | Svenstorp | Myotis daubentonii | male | adult |
| Skåne/2009 | Svenstorp | Myotis daubentonii | female | adult |
| Skåne/2009 | Svenstorp | Myotis daubentonii | female | adult |
| Skåne/2009 | Svenstorp | Myotis daubentonii | male | juvenile |
| Skåne/2009 | Stockamöllan | Myotis daubentonii | female | adult |
| Skåne/2009 | Ellinge | Myotis daubentonii | female | adult |
| Skåne/2009 | Ellinge | Myotis daubentonii | female | juvenile |
| Småland/2012 | Vassmolösa | Myotis daubentonii | female | adult |
| Småland/2012 | Vassmolösa | Myotis daubentonii | male | adult |
| Småland/2012 | Vassmolösa | Myotis daubentonii | male | adult |
| Småland/2012 | Vassmolösa | Myotis daubentonii | female | adult |
| Småland/2012 | Vassmolösa | Myotis daubentonii | female | adult |
| Småland/2012 | Vassmolösa | Myotis daubentonii | female | juvenile |
All 243 bats (Daubenton's and Northern bats) collected in central Sweden were found negative, while positive Daubenton's bats were found both in southern Sweden in 2009 and southeastern Sweden in 2012. In addition, two samples from 2012, also collected from Daubenton's bats in southeastern Sweden, were tested as borderlines (0.35 IE/ml, data not shown).
The prevalence of Daubenton's bats positive for EBLV reactive antibodies varied between 0% (0/32 in 2008 and 0/38 in 2013) and 10.3% (8/77 in 2009) in Skåne, and was 8.8% (6/68) in Småland in 2012.
Discussion
The surveillance of bat lyssaviruses has been varying among the different countries in Europe (23). Passive surveillance might be sufficient for revealing the required information on the occurrence of bat rabies. However, the total number of bats enclosed in passive surveillance obviously should be high, as shown by the so far completely negative results from Sweden. One significant obstacle by passive surveillance for bat rabies virus is that the major host for EBLV-2, Daubenton's bats, does not usually roost in houses, reducing the chance of the owners finding bats of this species.
For example, only 8 out of 199, 111 out of 3,873, and 144 out of 7,457 collected bats in passive surveillance projects in Finland, Netherlands, and UK, respectively, were of this bat species (10, 27, 28).
Active sampling by oral swabs has only scarcely resulted in positive findings of bat rabies virus, and our findings are also in line with this. More than 450 oral swabs were collected in this study, and all were found negative for EBLV RNA. More than 900 and 148 oral swabs have earlier been examined in Scotland and Switzerland, respectively, with only one EBLV-2 RNA positive Daubenton's bat detected in each country (23). No virus RNA was detected in 766 and 218 swabs, respectively, in two other studies from the UK (22, 29), and none in 774 and 124 swabs, respectively, in two separate studies from Finland (14, 30).
In contrast, antibodies neutralizing lyssavirus have been found in a number of various bat species in the UK, Germany, Switzerland, Finland, Spain, and Norway (14, 15, 31).
Such serological analyses are reliable indicators of past exposures to lyssavirus in bats. Seropositive Daubenton's bats have been reported from the UK, Finland, Switzerland (14, 23), and now also in Sweden. Because of the broad serological cross-reactivity between EBLV-1 and EBLV-2 (32–35), the specificity of the detected virus neutralization antibodies (VNAs) cannot be established. However, all of the seropositive bats are Daubenton's bats, which makes it likely to assume that the detected VNAs are a result of an infection with EBLV-2.
One infected Daubenton's bat was recently observed in Norway (15). The results from the present surveillance indicated that the seroprevalence of bat lyssavirus-specific antibodies in the Swedish Daubenton's bat population is low (0–8.8%), and they are similar with the findings of previous studies from the Netherlands (1.1–4.1% and 3.0–9.1), Finland (1.12–3.36%), and Switzerland (2.4%).
To date, 19 different bat species have been recorded in Sweden, out of a total 45 species known to be present in Europe (36). All these 19 species, except for the Northern bat, are in Sweden at the northern-most limit of their geographical range, and most of the species are restricted only to the southern part of the country. This study included mainly Daubenton's bats and Northern bats, and a few bats of three other species, requiring that many species still remain to be investigated for EBLV.
In contrast to the recorded low seroprevalences of antibodies presumably to EBLV-2, the seroprevalences to EBLV-1 in Spain and to RABV in the New World have been found relatively high (37).
The risk for the general public, without any contact to bats via work or leisure, is believed to be negligible. However, bat species resident in Sweden can be infected not only by EBLV but also with other lyssaviruses, including viruses not yet identified. The geographical distribution of various bat species in Sweden is not very well known based on the limited number of studies so far performed in the country.
All lyssavirus-infected bats so far detected in Sweden are Daubenton's bats (Fig. 1), which is a small and widespread species common in Eurasia. Daubenton's bat is described as a facultative seasonal migrant, covering middle-range distances between winter and summer roosts, usually within 100–150 km (38). It has been shown that especially females of Daubenton's bats move their roost quite often, usually within only a few days (39). Daubenton's bats live in ‘fusion-fission colonies’, in which the bats in the same roosting refuge form a ‘loose colony’ (40). The ‘fused colony’ is built by several subgroups, while fission occurs at the time of roost switching by subgroups breaking apart and mixing, ending up in various new roosts (41). This strategy has several ecological advantages, but unfortunately it also facilitates the efficient spreading of infections.
In this study, we have shown that bat lyssavirus infection is likely to be endemic in southern/southeastern Sweden. So far, passive surveillance has been negative in Sweden, and the number of bats sent in for passive surveillance should therefore be higher for a sufficient and relevant base of the occurrence of bat rabies virus in this country. The present results, however, indicated that a low, but significant, prevalence of EBLV is present in the Swedish Daubenton's bat population.
Acknowledgements
This study was partly supported by the Swedish board of Agriculture. This study had not been possible without the skillful help from Johnny de Jong, Anders Lindström, Johan Wallgren and other members of the staff at SVA, FoHM (former SMI) and Uppsala University.
References
- 1.Dietzgen RG, Calisher CH, Kurath G, Kuzmin IV, Rodriguez LL, Stone DM, et al. Family Rhabdooviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2011. pp. 654–681. [Google Scholar]
- 2.Jakava-Viljanen M, Nokireki T, Sironen T, Vapalahti O, Shone L, Huovilainen A. Evolutionary trends of European bat lyssavirus type 2 including genetic characterization of Finnish strains of human and bat origin 24 years apart. Arch Virol. 2015;160:1489–98. doi: 10.1007/s00705-015-2424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceballos NA, Morón SV, Berciano JM, Nicolás O, López CA, Juste J, et al. Novel lyssavirus in bat, Spain. Emerg Infect Dis. 2013;19:793–5. doi: 10.3201/eid1905.121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badrane H, Bahloud C, Perrin P, Tordo N. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J Virol. 2001;75:3268–76. doi: 10.1128/JVI.75.7.3268-3276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuzmin IV, Mayer AE, Niezgoda M, Markotter W, Agwanda B, Breiman RF, et al. Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res. 2010;149:197–210. doi: 10.1016/j.virusres.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Picard-Meyer E, Servat A, Robardet E, Moinet M, Borel C, Cliquet F. Isolation of Bokelo bat lyssavirus in Myotis nattereri in France. Arch Virol. 2013;158:2333–40. doi: 10.1007/s00705-013-1747-y. [DOI] [PubMed] [Google Scholar]
- 7.Bourhy H, Kissi B, Lafon M, Sacramento D, Tordo N. Antigenic and molecular characterization of bat rabies virus in Europe. J Clin Microbiol. 1992;30:2419–26. [Google Scholar]
- 8.WHO. Rabies Bulletin Europe. Available from: http://www.who-rabies-bulletin.org [cited 2 November 2016].
- 9.Fooks AR, Brookes SM, Johnson N, McElhinney LM, Hutson AM. European bat lyssaviruses: an emerging zoonosis. Epidemiol Infect. 2003;131:1029–39. doi: 10.1017/s0950268803001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris SL, Mansfield K, Marston DA, Johnson N, Pajamo K, O'brien N, et al. Isolation of European bat lyssavirus type 2 from a Daubenton's bat (Myotis daubentonii) in Shropshire. Vet Rec. 2007;161:384–6. doi: 10.1136/vr.161.11.384. [DOI] [PubMed] [Google Scholar]
- 11.Müller T, Cox J, Schafer R, Johnson N, McElhinney LM, Geue JL, et al. Spill-over of European bat lyssavirus type 1 into a stone marten (Martes foina) in Germany. J Vet Med B Infect Dis Vet Public Health. 2004;51:49–54. doi: 10.1111/j.1439-0450.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 12.Tjørnehøj K, Fooks AR, Agerholm JS, Rønsholt L. Natural and experimental infection of sheep with European bat lyssavirus type-1 of Danish bat origin. J Comp Pathol. 2006;134:190–201. doi: 10.1016/j.jcpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.McElhinney LM, Marston DA, Leech S, Freuling CM, van der Poel WH, Echevarria J, et al. Molecular epidemiology of bat lyssaviruses in Europe. Zoonoses Public Health. 2013;60:35–45. doi: 10.1111/zph.12003. [DOI] [PubMed] [Google Scholar]
- 14.Nokireki T, Huovilainen A, Lilley T, Kyheröinen E-M, Ek-Kommonen C, Sihvonen L, et al. Bat rabies surveillance in Finland. BMC Vet Res. 2013;9:174. doi: 10.1186/1746-6148-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaggermusrabies-pavist-i-Norge. Available from: http://www.miljodirektoratet.no/no/Nyheter/Nyheter/2015/Oktober-2015 [cited 2 November 2016].
- 16.Schneider LG, Cox JH. Bat lyssaviruses in Europe. Curr Top Microbiol Immunol. 1994;187:207–18. doi: 10.1007/978-3-642-78490-3_12. [DOI] [PubMed] [Google Scholar]
- 17.Fooks AR, McElhinney LM, Pounder DJ, Finnegan CJ, Mansfield K, Johnson N, et al. Case report: isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J Med Virol. 2003;71:281–9. doi: 10.1002/jmv.10481. [DOI] [PubMed] [Google Scholar]
- 18.Mollgard S. Bat rabies in Denmark. Rabies Bull Europe. 1985;9:8–11. [Google Scholar]
- 19.Lumio J, Hillbom M, Roine R, Ketonen L, Haltia M, Valle M, et al. Human rabies of bat origin in Europe. Lancet. 1986;15:378. doi: 10.1016/s0140-6736(86)92336-6. [DOI] [PubMed] [Google Scholar]
- 20.Dean DJ, Abelseth MK, Atanasui P. The fluorescent antibody test. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization; 1996. pp. 88–95. [Google Scholar]
- 21.Echevarria JE, Avellón A, Juste J, Vera M, Ibáñez C. Screening of active lyssavirus infection in wild bat populations by viral RNA detection on oropharyngeal swabs. J Clin Microbiol. 2001;39:3678–83. doi: 10.1128/JCM.39.10.3678-3683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brookes SM, Aegerter JN, Smith GC, Healy DM, Joliffe TA, Swift SM, et al. European bat Lyssavirus in Scottish bats. Emerg Infect Dis. 2005;4:572–8. doi: 10.3201/eid1104.040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatz J, Fooks AR, McElhinney I, Horton D, Echevarria J, Vazquez-Moron S, et al. Bat rabies surveillance in Europe. Zoonoses Public Health. 2013;60:22–34. doi: 10.1111/zph.12002. [DOI] [PubMed] [Google Scholar]
- 24.Heaton PR, Johnstone P, McElhinney LM, Cowley R, O'Sullivan E, Whitby JE. Heminested PCR assay for detection of six genotypes of rabies and rabies-related viruses. J Clin Microbiol. 1997;35:2762–6. doi: 10.1128/jcm.35.11.2762-2766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakeley PR, Johnson N, McElhinney LM, Marston D, Sawyer J, Fooks AR. Development of a real-time, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5, and 6. J Clin Microbiol. 2005;43:2786–92. doi: 10.1128/JCM.43.6.2786-2792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.OIE, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 2.1.17, – Rabies (infection with rabies virus). Section B adopted by the World Assembly of Delegates of the OIE in May 2011. [Google Scholar]
- 27.Jakava-Viljanen M, Lilley T, Kyheröinen E-M, Huovilainen A. First encounter of European bat lyssavirus type 2 (EBLV-2) in a bat in Finland. Epidemiol Infect. 2010;138:1581–5. doi: 10.1017/S0950268810000373. [DOI] [PubMed] [Google Scholar]
- 28.Van der Poel VHM, Van der Heide R, Verstraten E, Takumi K, Lina PHC, Kramps JA. European bat lyssaviruses, the Netherlands. Emerg Infect Dis. 2005;11:1854–9. doi: 10.3201/eid1112.041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris SL, Aegerter JN, Brookes SM, McElhinney LM, Jones G, Smith GC, Fooks AR. Targeted surveillance for European bat lyssaviruses in English bats (2003–06) J Wildl Dis. 2009;45:1030–41. doi: 10.7589/0090-3558-45.4.1030. [DOI] [PubMed] [Google Scholar]
- 30.Hagner NC, Kommonen E, Lokki E, Neuvonen T, Stjernberg T, Valle M, et al. No bat rabies found in Finland 1986. Eur Bat Res. 1887:631–5. [Google Scholar]
- 31.Vázquez-Morón S, Juste J, Ibáñez C, Ruiz-Villamor E, Avellón A, Vera M, et al. Endemic circulation of European bat lyssavirus type 1 in serotine bats, Spain. Emerg Infect Dis. 2008;14:1263–6. doi: 10.3201/1408.080068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brookes SM, Parsons G, Johnson N, McElhinney LM, Fooks AR. Rabies human diploid cell vaccine elicits cross-neutralising and cross-protecting immune responses against European and Australian bat lyssaviruses. Vaccine. 2005;32:4101–9. doi: 10.1016/j.vaccine.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Hanlon CA, Kuzmin IV, Blanton JD, Weldon WC, Manangan JS, Rupprecht CE. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005;111:44–54. doi: 10.1016/j.virusres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Müller T, Selhorst T, Burow J, Schameitat A, Vos A. Cross reactive antigenicity in orally vaccinated foxes and raccoon dogs against European Bat Lyssavirus type 1 and 2. Dev Biol (Basel) 2006;125:195–204. [PubMed] [Google Scholar]
- 35.Wright E, Temperton NJ, Marston DA, McElhinney LM, Fooks AR, Weiss RA. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J Gen Virol. 2008;89:2204–13. doi: 10.1099/vir.0.2008/000349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bat life Europe annual report. 2011/2012. Available from: http://www.batlife-europé-info/_webedit/uploaded files/Al Files/BatLife Europe Annual Report.pdf [cited 2 November 2016].
- 37.Franka R, Johnson N, Muller T, Vos A, Neubert L, Freuling C, et al. Susceptibility of North American big brown bats (Eptesicus fuscus) to infection with European bat lyssavirus type 1. J Gen Virol. 2008;89:1998–2010. doi: 10.1099/vir.0.83688-0. [DOI] [PubMed] [Google Scholar]
- 38.Hutterer R, Ivanova T, Meyer-Cords R, Rodrigues L. Bat migrations in Europe. A review on banding data and literature. Naturschutz und Biologische Vielfalt Heft 28. Bonn, Germany: Federal Agency for Nature Conservation; 2008. p. 162. [Google Scholar]
- 39.Lucan RK, Radil J. Variability of foraging and roosting activities in adult females of Daubenton's bat (Myotis daubentonii) in different seasons. Biologia. 2010;65:1072–80. [Google Scholar]
- 40.Kerth G. Causes and consequences of sociality in bats. Bioscience. 2008;58:737–46. [Google Scholar]
- 41.Kerth G, Van Schaik J. Causes and consequences of living in closed societies: lessons from a long-term socio-genetic study on Bechstein's bats. Mol Ecol. 2012;21:633–46. doi: 10.1111/j.1365-294X.2011.05233.x. [DOI] [PubMed] [Google Scholar]